Label: ITRACONAZOLE capsule
-
NDC Code(s):
46708-204-04,
46708-204-10,
46708-204-30,
46708-204-71, view more46708-204-90
- Packager: Alembic Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
BOXED WARNING
Congestive Heart Failure, Cardiac Effects and Drug Interactions:
Itraconazole capsules should not be administered for the treatment of onychomycosis in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF. If signs or symptoms of congestive heart failure occur during administration of itraconazole capsules, discontinue administration. When itraconazole was administered intravenously to dogs and healthy human volunteers, negative inotropic effects were seen. (See CONTRAINDICATIONS, WARNINGS, PRECAUTIONS: Drug Interactions, ADVERSE REACTIONS: Post-marketing Experience, and CLINICAL PHARMACOLOGY: Special Populations for more information.)
Drug Interactions: Coadministration of the following drugs are contraindicated with itraconazole capsules: methadone, disopyramide, dofetilide, dronedarone, quinidine, ergot alkaloids (such as dihydroergotamine, ergometrine (ergonovine), ergotamine, methylergometrine (methylergonovine)), irinotecan, lurasidone, oral midazolam, pimozide, triazolam, felodipine, nisoldipine, ranolazine, eplerenone, cisapride, lovastatin, simvastatin, ticagrelor and, in subjects with varying degrees of renal or hepatic impairment, colchicine, fesoterodine, telithromycin and solifenacin. See PRECAUTIONS: Drug Interactions Section for specific examples. Coadministration with itraconazole can cause elevated plasma concentrations of these drugs and may increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs. For example, increased plasma concentrations of some of these drugs can lead to QT prolongation and ventricular tachyarrhythmias including occurrences of torsades de pointes, a potentially fatal arrhythmia. See CONTRAINDICATIONS and WARNINGS Sections, and PRECAUTIONS: Drug Interactions Section for specific examples.
-
DESCRIPTION
Itraconazole Capsule is the generic name for itraconazole, an azole antifungal agent. Itraconazole is a 1:1:1:1 racemic mixture of four diastereomers (two enantiomeric pairs), each possessing three chiral centers. It may be represented by the following structural formula and nomenclature:
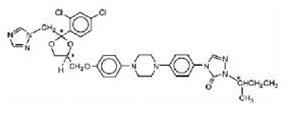
±)-1-[(R*)-sec-butyl]-4-[p-[4-[p-[[(2R*,4S*)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-piperazinyl]phenyl]-Δ2-1,2,4-triazolin-5-one mixture with (±)-1-[(R*)-sec-butyl]-4-[p-[4-[p-[[(2S*,4R*)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-piperazinyl]phenyl]-Δ2-1,2,4-triazolin-5-one
or
(±)-1-[(RS)-sec-butyl]-4-[p-[4-[p-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-piperazinyl]phenyl]-Δ2-1,2,4-triazolin-5-one
Itraconazole has a molecular formula of C35H38Cl2N8O4 and a molecular weight of 705.64. It is a white or almost white powder. It is freely soluble in methylene chloride, sparingly soluble in tetrahydrofuran, very slightly soluble in alcohol and practically insoluble in water. It has a pKa of 3.7 (based on extrapolation of values obtained from methanolic solutions) and a log (n-octanol/water) partition coefficient of 5.66 at pH 8.1.
Itraconazole capsules contain 100 mg of itraconazole coated on sugar spheres (composed of sucrose, starch and purified water). Inactive ingredients are hydroxypropyl methylcellulose, polyethylene glycol, hypromellose, gelatin, titanium dioxide, D&C Red No. 22, FD&C Blue No. 1, and D&C Red No. 28.
The imprinting ink contains shellac, propylene glycol, black iron oxide, and potassium hydroxide.
-
CLINICAL PHARMACOLOGY
General Pharmacokinetic Characteristics
Peak plasma concentrations of itraconazole are reached within 2 to 5 hours following oral administration. As a consequence of non-linear pharmacokinetics, itraconazole accumulates in plasma during multiple dosing. Steady-state concentrations are generally reached within about 15 days, with Cmax values of 0.5 μg/ml, 1.1 μg/ml and 2 μg/ml after oral administration of 100 mg once daily, 200 mg once daily and 200 mg b.i.d., respectively. The terminal half-life of itraconazole generally ranges from 16 to 28 hours after single dose and increases to 34 to 42 hours with repeated dosing. Once treatment is stopped, itraconazole plasma concentrations decrease to an almost undetectable concentration within 7 to 14 days, depending on the dose and duration of treatment. Itraconazole mean total plasma clearance following intravenous administration is 278 ml/min. Itraconazole clearance decreases at higher doses due to saturable hepatic metabolism.
Absorption
Itraconazole is rapidly absorbed after oral administration. Peak plasma concentrations of itraconazole are reached within 2 to 5 hours following an oral capsule dose. The observed absolute oral bioavailability of itraconazole is about 55%.
The oral bioavailability of itraconazole is maximal when itraconazole capsules are taken immediately after a full meal. Absorption of itraconazole capsules is reduced in subjects with reduced gastric acidity, such as subjects taking medications known as gastric acid secretion suppressors (e.g., H2-receptor antagonists, proton pump inhibitors) or subjects with achlorhydria caused by certain diseases. (See PRECAUTIONS: Drug Interactions.) Absorption of itraconazole under fasted conditions in these subjects is increased when itraconazole capsules are administered with an acidic beverage (such as a non-diet cola). When itraconazole capsules were administered as a single 200-mg dose under fasted conditions with non-diet cola after ranitidine pretreatment, a H2-receptor antagonist, itraconazole absorption was comparable to that observed when itraconazole capsules were administered alone. (See PRECAUTIONS: Drug Interactions.)
Itraconazole exposure is lower with the Capsule formulation than with the Oral Solution when the same dose of drug is given. (See WARNINGS)
Distribution
Most of the itraconazole in plasma is bound to protein (99.8%), with albumin being the main binding component (99.6% for the hydroxy-metabolite). It has also a marked affinity for lipids. Only 0.2% of the itraconazole in plasma is present as free drug. Itraconazole is distributed in a large apparent volume in the body (>700 L), suggesting extensive distribution into tissues. Concentrations in lung, kidney, liver, bone, stomach, spleen and muscle were found to be two to three times higher than corresponding concentrations in plasma, and the uptake into keratinous tissues, skin in particular, up to four times higher. Concentrations in the cerebrospinal fluid are much lower than in plasma.
Metabolism
Itraconazole is extensively metabolized by the liver into a large number of metabolites. In vitro studies have shown that CYP3A4 is the major enzyme involved in the metabolism of itraconazole. The main metabolite is hydroxy-itraconazole, which has in vitro antifungal activity comparable to itraconazole; trough plasma concentrations of this metabolite are about twice those of itraconazole.
Excretion
Itraconazole is excreted mainly as inactive metabolites in urine (35%) and in feces (54%) within one week of an oral solution dose. Renal excretion of itraconazole and the active metabolite hydroxy-itraconazole account for less than 1% of an intravenous dose. Based on an oral radiolabeled dose, fecal excretion of unchanged drug ranges from 3% to 18% of the dose.
As re-distribution of itraconazole from keratinous tissues appears to be negligible, elimination of itraconazole from these tissues is related to epidermal regeneration. Contrary to plasma, the concentration in skin persists for 2 to 4 weeks after discontinuation of a 4-week treatment and in nail keratin – where itraconazole can be detected as early as 1 week after start of treatment – for at least six months after the end of a 3-month treatment period.
Special Populations:
Renal Impairment:
Limited data are available on the use of oral itraconazole in patients with renal impairment. A pharmacokinetic study using a single 200-mg oral dose of itraconazole was conducted in three groups of patients with renal impairment (uremia: n=7; hemodialysis: n=7; and continuous ambulatory peritoneal dialysis: n=5). In uremic subjects with a mean creatinine clearance of 13 mL/min. × 1.73 m2, the exposure, based on AUC, was slightly reduced compared with normal population parameters. This study did not demonstrate any significant effect of hemodialysis or continuous ambulatory peritoneal dialysis on the pharmacokinetics of itraconazole (Tmax, Cmax, and AUC0-8h). Plasma concentration-versus-time profiles showed wide intersubject variation in all three groups. After a single intravenous dose, the mean terminal half-lives of itraconazole in patients with mild (defined in this study as CrCl 50 to 79 ml/min), moderate (defined in this study as CrCl 20 to 49 ml/min), and severe renal impairment (defined in this study as CrCl <20 ml/min) were similar to that in healthy subjects (range of means 42 to 49 hours vs 48 hours in renally impaired patients and healthy subjects, respectively). Overall exposure to itraconazole, based on AUC, was decreased in patients with moderate and severe renal impairment by approximately 30% and 40%, respectively, as compared with subjects with normal renal function. Data are not available in renally impaired patients during long-term use of itraconazole. Dialysis has no effect on the half-life or clearance of itraconazole or hydroxyl-itraconazole. (See PRECAUTIONS and DOSAGE AND ADMINISTRATION.)
Hepatic Impairment:
Itraconazole is predominantly metabolized in the liver. A pharmacokinetic study was conducted in 6 healthy and 12 cirrhotic subjects who were administered a single 100-mg dose of itraconazole as capsule. A statistically significant reduction in mean Cmax (47%) and a twofold increase in the elimination half-life (37 ± 17 hours vs. 16 ± 5 hours) of itraconazole were noted in cirrhotic subjects compared with healthy subjects. However, overall exposure to itraconazole, based on AUC, was similar in cirrhotic patients and in healthy subjects. Data are not available in cirrhotic patients during long-term use of itraconazole. (See CONTRAINDICATIONS, PRECAUTIONS: Drug Interactions and DOSAGE AND ADMINISTRATION.)
Decreased Cardiac Contractility:
When itraconazole was administered intravenously to anesthetized dogs, a dose-related negative inotropic effect was documented. In a healthy volunteer study of itraconazole intravenous infusion, transient, asymptomatic decreases in left ventricular ejection fraction were observed using gated SPECT imaging; these resolved before the next infusion, 12 hours later. If signs or symptoms of congestive heart failure appear during administration of itraconazole capsules, itraconazole should be discontinued. (See BOXED WARNING, CONTRAINDICATIONS, WARNINGS, PRECAUTIONS: Drug Interactions and ADVERSE REACTIONS: Post-marketing Experience for more information.)
MICROBIOLOGY
Mechanism of Action:
In vitro studies have demonstrated that itraconazole inhibits the cytochrome P450-dependent synthesis of ergosterol, which is a vital component of fungal cell membranes.
Activity In Vitro and in Clinical Infections:
Itraconazole exhibits in vitro activity against Blastomyces dermatitidis, Histoplasma capsulatum, Histoplasma duboisii, Aspergillus flavus, Aspergillus fumigatus, and Trichophyton species (See INDICATIONS AND USAGE, Description of Clinical Studies).
Correlation between minimum inhibitory concentration (MIC) results in vitro and clinical outcome has yet to be established for azole antifungal agents.
Drug Resistance:
Isolates from several fungal species with decreased susceptibility to itraconazole have been isolated in vitro and from patients receiving prolonged therapy.
Itraconazole is not active against Zygomycetes (e.g., Rhizopus spp., Rhizomucor spp., Mucor spp. and Absidia spp.), Fusarium spp., Scedosporium spp. and Scopulariopsis spp.
Cross-resistance:
Several in vitro studies have reported that some fungal clinical isolates with reduced susceptibility to one azole antifungal agent may also be less susceptible to other azole derivatives. The finding of cross-resistance is dependent on a number of factors, including the species evaluated, its clinical history, the particular azole compounds compared, and the type of susceptibility test that is performed.
Studies (both in vitro and in vivo) suggest that the activity of amphotericin B may be suppressed by prior azole antifungal therapy. As with other azoles, itraconazole inhibits the 14C-demethylation step in the synthesis of ergosterol, a cell wall component of fungi. Ergosterol is the active site for amphotericin B. In one study the antifungal activity of amphotericin B against Aspergillus fumigatus infections in mice was inhibited by ketoconazole therapy. The clinical significance of test results obtained in this study is unknown.
-
INDICATIONS AND USAGE
Itraconazole capsules are indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients:
1. Blastomycosis, pulmonary and extrapulmonary
2. Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non- meningeal histoplasmosis, and
3. Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy.
Specimens for fungal cultures and other relevant laboratory studies (wet mount, histopathology, serology) should be obtained before therapy to isolate and identify causative organisms. Therapy may be instituted before the results of the cultures and other laboratory studies are known; however, once these results become available, antiinfective therapy should be adjusted accordingly.
Itraconazole capsules are also indicated for the treatment of the following fungal infections in non-immunocompromised patients:
1. Onychomycosis of the toenail, with or without fingernail involvement, due to dermatophytes (tinea unguium), and
2. Onychomycosis of the fingernail due to dermatophytes (tinea unguium).
Prior to initiating treatment, appropriate nail specimens for laboratory testing (KOH preparation, fungal culture, or nail biopsy) should be obtained to confirm the diagnosis of onychomycosis.
(See CLINICAL PHARMACOLOGY: Special Populations, CONTRAINDICATIONS, WARNINGS, and ADVERSE REACTIONS: Post-marketing Experience for more information.)
Description of Clinical Studies:
Blastomycosis:
Analyses were conducted on data from two open-label, non-concurrently controlled studies (N=73 combined) in patients with normal or abnormal immune status. The median dose was 200 mg/day. A response for most signs and symptoms was observed within the first 2 weeks, and all signs and symptoms cleared between 3 and 6 months. Results of these two studies demonstrated substantial evidence of the effectiveness of itraconazole for the treatment of blastomycosis compared with the natural history of untreated cases.
Histoplasmosis:
Analyses were conducted on data from two open-label, non-concurrently controlled studies (N=34 combined) in patients with normal or abnormal immune status (not including HIV-infected patients). The median dose was 200 mg/day. A response for most signs and symptoms was observed within the first 2 weeks, and all signs and symptoms cleared between 3 and 12 months. Results of these two studies demonstrated substantial evidence of the effectiveness of itraconazole for the treatment of histoplasmosis, compared with the natural history of untreated cases.
Histoplasmosis in HIV-infected patients:
Data from a small number of HIV-infected patients suggested that the response rate of histoplasmosis in HIV-infected patients is similar to that of non-HIV-infected patients. The clinical course of histoplasmosis in HIV-infected patients is more severe and usually requires maintenance therapy to prevent relapse.
Aspergillosis:
Analyses were conducted on data from an open-label, “single-patient-use” protocol designed to make itraconazole available in the U.S. for patients who either failed or were intolerant of amphotericin B therapy (N=190). The findings were corroborated by two smaller open-label studies (N=31 combined) in the same patient population. Most adult patients were treated with a daily dose of 200 to 400 mg, with a median duration of 3 months. Results of these studies demonstrated substantial evidence of effectiveness of itraconazole as a second-line therapy for the treatment of aspergillosis compared with the natural history of the disease in patients who either failed or were intolerant of amphotericin B therapy.
Onychomycosis of the toenail:
Analyses were conducted on data from three double-blind, placebo-controlled studies (N=214 total; 110 given itraconazole capsules in which patients with onychomycosis of the toenails received 200 mg of itraconazole capsules once daily for 12 consecutive weeks. Results of these studies demonstrated mycologic cure, defined as simultaneous occurrence of negative KOH plus negative culture, in 54% of patients. Thirty-five percent (35%) of patients were considered an overall success (mycologic cure plus clear or minimal nail involvement with significantly decreased signs) and 14% of patients demonstrated mycologic cure plus clinical cure (clearance of all signs, with or without residual nail deformity). The mean time to overall success was approximately 10 months. Twenty-one percent (21%) of the overall success group had a relapse (worsening of the global score or conversion of KOH or culture from negative to positive).
Onychomycosis of the fingernail:
Analyses were conducted on data from a double-blind, placebo-controlled study (N=73 total; 37 given itraconazole capsules in which patients with onychomycosis of the fingernails received a 1-week course of 200 mg of itraconazole capsules b.i.d., followed by a 3-week period without itraconazole, which was followed by a second 1-week course of 200 mg of itraconazole capsules b.i.d. Results demonstrated mycologic cure in 61% of patients. Fifty-six percent (56%) of patients were considered an overall success and 47% of patients demonstrated mycologic cure plus clinical cure. The mean time to overall success was approximately 5 months. None of the patients who achieved overall success relapsed.
-
CONTRAINDICATIONS
Congestive Heart Failure:
Itraconazole capsules should not be administered for the treatment of onychomycosis in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF. (See BOXED WARNINGS, WARNINGS, PRECAUTIONS: Drug Interactions-Calcium Channel Blockers, ADVERSE REACTIONS: Post-marketing Experience, and CLINICAL PHARMACOLOGY: Special Populations.)
Drug Interactions:
Coadministration of a number of CYP3A4 substrates are contraindicated with itraconazole. Plasma concentrations increase for the following drugs: methadone, disopyramide, dofetilide, dronedarone, quinidine, ergot alkaloids (such as dihydroergotamine, ergometrine (ergonovine), ergotamine, methylergometrine (methylergonovine)), irinotecan, lurasidone, oral midazolam, pimozide, triazolam, felodipine, nisoldipine, ranolazine, eplerenone, cisapride, lovastatin, simvastatin, ticagrelor, and, in subjects with varying degrees of renal or hepatic impairment, colchicine, fesoterodine, telithromycin and solifenacin. (See PRECAUTIONS: Drug Interactions Section for specific examples.) This increase in drug concentrations caused by coadministration with itraconazole may increase or prolong both the pharmacologic effect and/or adverse reactions to these drugs. For example, increased plasma concentrations of some of these drugs can lead to QT prolongation and ventricular tachyarrhythmias including occurrences of torsade de pointes, a potentially fatal arrhythmia. Specific examples are listed in PRECAUTIONS: Drug Interactions.
Itraconazole should not be administered for the treatment of onychomycosis to pregnant patients or to women contemplating pregnancy.
Itraconazole are contraindicated for patients who have shown hypersensitivity to itraconazole. There is limited information regarding cross-hypersensitivity between itraconazole and other azole antifungal agents. Caution should be used when prescribing itraconazole to patients with hypersensitivity to other azoles.
-
WARNINGS
Hepatic Effects:
Itraconazole have been associated with rare cases of serious hepatotoxicity, including liver failure and death. Some of these cases had neither pre-existing liver disease nor a serious underlying medical condition, and some of these cases developed within the first week of treatment. If clinical signs or symptoms develop that are consistent with liver disease, treatment should be discontinued and liver function testing performed. Continued itraconazole use or reinstitution of treatment with itraconazole is strongly discouraged unless there is a serious or life-threatening situation where the expected benefit exceeds the risk. (See PRECAUTIONS: Information for Patients and ADVERSE REACTIONS.)
Cardiac Dysrhythmias:
Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using drugs such as cisapride, pimozide, methadone, or quinidine concomitantly with itraconazole and/or other CYP3A4 inhibitors. Concomitant administration of these drugs with itraconazole is contraindicated. (See BOXED WARNING, CONTRAINDICATIONS, and PRECAUTIONS: Drug Interactions.)
Cardiac Disease:
Itraconazole capsules should not be administered for the treatment of onychomycosis in patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF. Itraconazole capsules should not be used for other indications in patients with evidence of ventricular dysfunction unless the benefit clearly outweighs the risk.
For patients with risk factors for congestive heart failure, physicians should carefully review the risks and benefits of itraconazole therapy. These risk factors include cardiac disease such as ischemic and valvular disease; significant pulmonary disease such as chronic obstructive pulmonary disease; and renal failure and other edematous disorders. Such patients should be informed of the signs and symptoms of CHF, should be treated with caution, and should be monitored for signs and symptoms of CHF during treatment. If signs or symptoms of CHF appear during administration of itraconazole capsules, discontinue administration.
Itraconazole has been shown to have a negative inotropic effect. When itraconazole was administered intravenously to anesthetized dogs, a dose-related negative inotropic effect was documented. In a healthy volunteer study of itraconazole intravenous infusion, transient, asymptomatic decreases in left ventricular ejection fraction were observed using gated SPECT imaging; these resolved before the next infusion, 12 hours later.
Itraconazole has been associated with reports of congestive heart failure. In post-marketing experience, heart failure was more frequently reported in patients receiving a total daily dose of 400 mg although there were also cases reported among those receiving lower total daily doses.
Calcium channel blockers can have negative inotropic effects which may be additive to those of itraconazole. In addition, itraconazole can inhibit the metabolism of calcium channel blockers. Therefore, caution should be used when co-administering itraconazole and calcium channel blockers due to an increased risk of CHF. Concomitant administration of itraconazole and felodipine or nisoldipine is contraindicated.
Cases of CHF, peripheral edema, and pulmonary edema have been reported in the post-marketing period among patients being treated for onychomycosis and/or systemic fungal infections. (See CLINICAL PHARMACOLOGY: Special Populations, CONTRAINDICATIONS, PRECAUTIONS: Drug Interactions, and ADVERSE REACTIONS: Post-marketing Experience for more information.)
Interaction potential:
Itraconazole has a potential for clinically important drug interactions. Coadministration of specific drugs with itraconazole may result in changes in efficacy of itraconazole and/or the coadministered drug, life-threatening effects and/or sudden death. Drugs that are contraindicated, not recommended or recommended for use with caution in combination with itraconazole are listed in PRECAUTIONS: Drug Interactions.
Interchangeability:
Itraconazole capsules and itraconazole Oral Solution should not be used interchangeably. This is because drug exposure is greater with the Oral Solution than with the capsules when the same dose of drug is given. In addition, the topical effects of mucosal exposure may be different between the two formulations. Only the Oral Solution has been demonstrated effective for oral and/or esophageal candidiasis.
-
PRECAUTIONS
General:
Itraconazole capsulesshould be administered after a full meal. (See CLINICAL PHARMACOLOGY: Pharmacokinetics and Metabolism).
Under fasted conditions, itraconazole absorption was decreased in the presence of decreased gastric acidity. The absorption of itraconazole may be decreased with the concomitant administration of antacids or gastric acid secretion suppressors. Studies conducted under fasted conditions demonstrated that administration with 8 ounces of a non-diet cola beverage resulted in increased absorption of itraconazole in AIDS patients with relative or absolute achlorhydria. This increase relative to the effects of a full meal is unknown. (See CLINICAL PHARMACOLOGY: Pharmacokinetics and Metabolism).
Hepatotoxicity:
Rare cases of serious hepatotoxicity have been observed with itraconazole treatment, including some cases within the first week. It is recommended that liver function monitoring be considered in all patients receiving itraconazole. Treatment should be stopped immediately and liver function testing should be conducted in patients who develop signs and symptoms suggestive of liver dysfunction.
Neuropathy:
If neuropathy occurs that may be attributable to itraconazole capsules, the treatment should be discontinued.
Cystic Fibrosis:
If a cystic fibrosis patient does not respond to itraconazole capsules, consideration should be given to switching to alternative therapy. For more information concerning the use of itraconazole in cystic fibrosis patients see the prescribing information for itraconazole Oral Solution.Hearing Loss:
Transient or permanent hearing loss has been reported in patients receiving treatment with itraconazole. Several of these reports included concurrent administration of quinidine which is contraindicated (see BOXED WARNING: Drug Interactions, CONTRAINDICATIONS: Drug Interactions and PRECAUTIONS: Drug Interactions). The hearing loss usually resolves when treatment is stopped, but can persist in some patients.
Information for Patients:
- The topical effects of mucosal exposure may be different between the itraconazole capsules and Oral Solution. Only the Oral Solution has been demonstrated effective for oral and/or esophageal candidiasis. Itraconazole capsules should not be used interchangeably with itraconazoleOral Solution.
- Instruct patients to take Itraconazole capsules with a full meal. Itraconazole capsules must be swallowed whole.
- Instruct patients about the signs and symptoms of congestive heart failure, and if these signs or symptoms occur during itraconazole administration, they should discontinue itraconazole and contact their healthcare provider immediately.
- Instruct patients to stop itraconazole treatment immediately and contact their healthcare provider if any signs and symptoms suggestive of liver dysfunction develop. Such signs and symptoms may include unusual fatigue, anorexia, nausea and/or vomiting, jaundice, dark urine, or pale stools.
- Instruct patients to contact their physician before taking any concomitant medications with itraconazole to ensure there are no potential drug interactions.
- Instruct patients that hearing loss can occur with the use of itraconazole. The hearing loss usually resolves when treatment is stopped, but can persist in some patients. Advise patients to discontinue therapy and inform their physicians if any hearing loss symptoms occur.
- Instruct patients that dizziness or blurred/double vision can sometimes occur with itraconazole. Advise patients that if they experience these events, they should not drive or use machines.
Drug Interactions:
Itraconazole is mainly metabolized through CYP3A4. Other drugs that either share this metabolic pathway or modify CYP3A4 activity may influence the pharmacokinetics of itraconazole. Similarly, itraconazole may modify the pharmacokinetics of other drugs that share this metabolic pathway. Itraconazole is a potent CYP3A4 inhibitor and a P-glycoprotein inhibitor. When using concomitant medication, it is recommended that the corresponding label be consulted for information on the route of metabolism and the possible need to adjust dosages.
Drugs that may decrease itraconazole plasma concentrations
Drugs that reduce the gastric acidity (e.g. acid neutralizing medicines such as aluminum hydroxide, or acid secretion suppressors such as H2-receptor antagonists and proton pump inhibitors) impair the absorption of itraconazole from itraconazole capsules. It is recommended that these drugs be used with caution when coadministered with itraconazole capsules:
· It is recommended that itraconazole capsules be administered with an acidic beverage (such as non-diet cola) upon co-treatment with drugs reducing gastric acidity.
· It is recommended that acid neutralizing medicines (e.g. aluminum hydroxide) be administered at least 1 hour before or 2 hours after the intake of itraconazole capsules.
· Upon coadministration, it is recommended that the antifungal activity be monitored and the itraconazole dose increased as deemed necessary.
Coadministration of itraconazole with potent enzyme inducers of CYP3A4 may decrease the bioavailability of itraconazole and hydroxy-itraconazole to such an extent that efficacy may be reduced. Examples include:
· Antibacterials: isoniazid, rifabutin (see also under ‘Drugs that may have their plasma concentrations increased by itraconazole’), rifampicin
· Anticonvulsants: carbamazepine, (see also under ‘Drugs that may have their plasma concentrations increased by itraconazole’), phenobarbital, phenytoin
· Antivirals: efavirenz, nevirapine
Therefore, administration of potent enzyme inducers of CYP3A4 with itraconazole is not recommended. It is recommended that the use of these drugs be avoided from 2 weeks before and during treatment with itraconazole, unless the benefits outweigh the risk of potentially reduced itraconazole efficacy. Upon coadministration, it is recommended that the antifungal activity be monitored and the itraconazole dose increased as deemed necessary.
Drugs that may increase itraconazole plasma concentrations
Potent inhibitors of CYP3A4 may increase the bioavailability of itraconazole. Examples include:
· Antibacterials: ciprofloxacin, clarithromycin, erythromycin
· Antivirals: ritonavir-boosted darunavir, ritonavir-boosted fosamprenavir, indinavir (see also under ‘Drugs that may have their plasma concentrations increased by itraconazole’), ritonavir (see also under ‘Drugs that may have their plasma concentrations increased by itraconazole’) and telaprevir.
It is recommended that these drugs be used with caution when coadministered with itraconazole capsules. It is recommended that patients who must take itraconazole concomitantly with potent inhibitors of CYP3A4 be monitored closely for signs or symptoms of increased or prolonged pharmacologic effects of itraconazole, and the itraconazole dose be decreased as deemed necessary.
Drugs that may have their plasma concentrations increased by itraconazole
Itraconazole and its major metabolite, hydroxy-itraconazole, can inhibit the metabolism of drugs metabolized by CYP3A4 and can inhibit the drug transport by P-glycoprotein, which may result in increased plasma concentrations of these drugs and/or their active metabolite(s) when they are administered with itraconazole. These elevated plasma concentrations may increase or prolong both therapeutic and adverse effects of these drugs. CYP3A4-metabolized drugs known to prolong the QT interval may be contraindicated with itraconazole, since the combination may lead to ventricular tachyarrhythmias including occurrences of torsade de pointes, a potentially fatal arrhythmia. Once treatment is stopped, itraconazole plasma concentrations decrease to an almost undetectable concentration within 7 to 14 days, depending on the dose and duration of treatment. In patients with hepatic cirrhosis or in subjects receiving CYP3A4 inhibitors, the decline in plasma concentrations may be even more gradual. This is particularly important when initiating therapy with drugs whose metabolism is affected by itraconazole.
Examples of drugs that may have their plasma concentrations increased by itraconazole presented by drug class with advice regarding coadministration with itraconazole:
Table 1: Drugs that may have their plasma concentrations increased by itraconazole
Drug Class
Contraindicated
Not Recommended
Use with Caution
Comments
Under no circumstances is the drug to be coadministered with itraconazole, and up to two weeks after discontinuation of treatment with itraconazole.
It is recommended that the use of the drug be avoided during and up to two weeks after discontinuation of treatment with itraconazole, unless the benefits outweigh the potentially increased risks of side effects. If coadministration cannot be avoided, clinical monitoring for signs or symptoms of increased or prolonged effects or side effects of the interacting drug is recommended, and its dosage be reduced or interrupted as deemed necessary. When appropriate, it is recommended that plasma concentrations be measured. The label of the coadministered drug should be consulted for information on dose adjustment and adverse effects.
Careful monitoring is recommended when the drug is coadministered with itraconazole. Upon coadministration, it is recommended that patients be monitored closely for signs or symptoms of increased or prolonged effects or side effects of the interacting drug, and its dosage be reduced as deemed necessary. When appropriate, it is recommended that plasma concentrations be measured. The label of the coadministered drug should be consulted for information on dose adjustment and adverse effects.
Alpha Blockers
tamsulosin
Analgesics
methadone
alfentanil,
buprenorphine IV and sublingual,
fentanyl,
oxycodone,
sufentanil
Methadone: The potential increase in plasma concentrations of methadone when coadministered with itraconazole may increase the risk of serious cardiovascular events including QTc prolongation and torsade de pointes.
Fentanyl: The potential increase in plasma concentrations of fentanyl when coadministered with itraconazole may increase the risk of potentially fatal respiratory depression.
Sufentanil: No human pharmacokinetic data of an interaction with itraconazole are available. In vitro data suggest that sufentanil is metabolized by CYP3A4 and so potentially increased sufentanil plasma concentrations would be expected when coadministered with itraconazole.
Antiarrhythmics
disopyramide,
dofetilide,
dronedarone,
quinidine
digoxin
Disopyramide, dofetilide, dronedarone, quinidine: The potential increase in plasma concentrations of these drugs when coadministered with itraconazole may increase the risk of serious cardiovascular events including QTc prolongation.
Antibacterials
telithromycin, in subjects with severe renal impairment or severe hepatic impairment
rifabutin
telithromycin
Telithromycin: The potential increase in plasma concentrations of telithromycin in subjects with severe renal impairment or severe hepatic impairment, when coadministered with itraconazole may increase the risk of serious cardiovascular events including QT prolongation and torsade de pointes.
Rifabutin: See also under ‘Drugs that may decrease itraconazole plasma concentrations’.
Anticoagulants and Antiplatelet Drugs
ticagrelor
apixaban,
rivaroxaban
coumarins,
cilostazol,
dabigatran
Ticagrelor: The potential increase in plasma concentrations of ticagrelor may increase the risk of bleeding.
Coumarins: Itraconazole may enhance the anticoagulant effect of coumarin-like drugs, such as warfarin.
Anticonvulsants
carbamazepine
Carbamazepine: In vivo studies have demonstrated an increase in plasma carbamazepine concentrations in subjects concomitantly receiving ketoconazole. Although there are no data regarding the effect of itraconazole on carbamazepine metabolism, because of the similarities between ketoconazole and itraconazole, concomitant administration of itraconazole and carbamazepine may inhibit the metabolism of carbamazepine. See also under ‘Drugs that may decrease itraconazole plasma concentrations’.
Antidiabetics
repaglinide,
saxagliptin
Antihelmintics and Antiprotozoals
praziquantel
Antimigraine Drugs
ergot alkaloids, such as dihydroergotamine, ergometrine (ergonovine), ergotamine, methylergometrine (methylergonovine)
eletriptan
Ergot Alkaloids: The potential increase in plasma concentrations of ergot alkaloids when coadministered with itraconazole may increase the risk of ergotism, i.e., a risk for vasospasm potentially leading to cerebral ischemia and/or ischemia of the extremities.
Antineoplastics
irinotecan
axitinib,
dabrafenib,
dasatinib,
ibrutinib,
nilotinib,
sunitinib
bortezomib,
busulphan,
docetaxel,
erlotinib,
imatinib,
ixabepilone,
lapatinib,
ponatinib,
trimetrexate,
vinca alkaloids
Irinotecan: The potential increase in plasma concentrations of irinotecan when coadministered with itraconazole may increase the risk of potentially fatal adverse events.
Antipsychotics,
Anxiolytics and Hypnotics
lurasidone,
oral midazolam,
pimozide,
triazolam
alprazolam,
aripiprazole,
buspirone,
diazepam,
haloperidol,
midazolam IV,
perospirone,
quetiapine,
ramelteon,
risperidone
Midazolam, triazolam:
Coadministration of itraconazole and oral midazolam, or triazolam may cause several-fold increases in plasma concentrations of these drugs. This may potentiate and prolong hypnotic and sedative effects, especially with repeated dosing or chronic administration of these agents.
Pimozide: The potential increase in plasma concentrations of pimozide when coadministered with itraconazole may increase the risk of serious cardiovascular events including QTc prolongation and torsade de pointes.
Antivirals
simeprevir
maraviroc,
indinavir,
ritonavir,
saquinavir
Indinavir, ritonavir: See also under ‘Drugs that may increase itraconazole plasma concentrations’.
Beta Blockers
nadolol
Calcium Channel
Blockers
felodipine,
nisoldipine
other dihydropyridines,
verapamil
Calcium channel blockers canhave a negative inotropic effect which may be additive to those of itraconazole. The potential increase in plasma concentrations of calcium channel blockers when co-administered with itraconazole may increase the risk of congestive heart failure.
Dihydropyridines: Concomitant administration of itraconazole may cause several-fold increases in plasma concentrations of dihydropyridines. Edema has been reported in patients concomitantly receiving itraconazole and dihydropyridine calcium channel blockers.
Cardiovascular Drugs, Miscellaneous
ranolazine
aliskiren,
sildenafil, for the treatment of pulmonary hypertension
bosentan,
riociguat
Ranolazine: The potential increase in plasma concentrations of ranolazine when coadministered with itraconazole may increase the risk of serious cardiovascular events including QTc prolongation.
Diuretics
eplerenone
Eplerenone: The potential increase in plasma concentrations of eplerenone when coadministered with itraconazole may increase the risk of hyperkalemia and hypotension.
Gastrointestinal Drugs
cisapride
aprepitant
Cisapride: The potential increase in plasma concentrations of cisapride when coadministered with itraconazole may increase the risk of serious cardiovascular events including QTc prolongation.
Immunosuppres
sants
everolimus,
temsirolimus
budesonide,
ciclesonide,
cyclosporine,
dexamethasone,
fluticasone, methylprednisolone,
rapamycin (also known as sirolimus),
tacrolimus
Lipid Regulating Drugs
lovastatin,
simvastatin
atorvastatin
The potential increase in plasma concentrations of atorvastatin, lovastatin, and simvastatin when coadministered with itraconazole may increase the risk of skeletal muscle toxicity, including rhabdomyolysis.
Respiratory Drugs
salmeterol
Urological Drugs
fesoterodine, in subjects with moderate to severe renal impairment, or moderate to severe hepatic impairment, solifenacin, in subjects with severe renal impairment or moderate to severe hepatic impairment
darifenacin,
vardenafil
fesoterodine.
oxybutynin,
sildenafil, for the treatment of erectile dysfunction,
solifenacin,
tadalafil,
tolterodine
Fesoterodine: The potential increase in plasma concentrations of the fesoterodine active metabolite may be greater in subjects with moderate to severe renal impairment, or moderate to severe hepatic impairment, which may lead to an increased risk of adverse reactions.
Solifenacin: The potential increase in plasma concentrations of solifenacin in subjects with severe renal impairment or moderate to severe hepatic impairment, when coadministered with itraconazole may increase the risk of serious cardiovascular events including QT prolongation.
Other
colchicine, in subjects with renal or hepatic impairment
colchicine, conivaptan, tolvaptan
cinacalcet
Colchicine: The potential increase in plasma concentrations of colchicine when coadministered with itraconazole may increase the risk of potentially fatal adverse events.
Conivaptan and Tolvaptan: A safe and effective dose of either conivaptan or tolvaptan has not been established when coadministered with itraconazole.
Drugs that may have their plasma concentrations decreased by itraconazole
Coadministration of itraconazole with the NSAID meloxicam may decrease the plasma concentration of meloxicam. It is recommended that meloxicam be used with caution when coadministered with itraconazole, and its effects or side effects be monitored. It is recommended that the dosage of meloxicam, if coadministered with itraconazole, be adjusted if necessary.Pediatric Population
Interaction studies have only been performed in adults.Carcinogenesis, Mutagenesis, and Impairment of Fertility:
Itraconazole showed no evidence of carcinogenicity potential in mice treated orally for 23 months at dosage levels up to 80 mg/kg/day (approximately 10x the maximum recommended human dose [MRHD]). Male rats treated with 25 mg/kg/day (3.1x MRHD) had a slightly increased incidence of soft tissue sarcoma. These sarcomas may have been a consequence of hypercholesterolemia, which is a response of rats, but not dogs or humans, to chronic itraconazole administration. Female rats treated with 50 mg/kg/day (6.25x MRHD) had an increased incidence of squamous cell carcinoma of the lung (2/50) as compared to the untreated group. Although the occurrence of squamous cell carcinoma in the lung is extremely uncommon in untreated rats, the increase in this study was not statistically significant.
Itraconazole produced no mutagenic effects when assayed in DNA repair test (unscheduled DNA synthesis) in primary rat hepatocytes, in Ames tests with Salmonella typhimurium (6 strains) and Escherichia coli, in the mouse lymphoma gene mutation tests, in a sex-linked recessive lethal mutation (Drosophila melanogaster) test, in chromosome aberration tests in human lymphocytes, in a cell transformation test with C3H/10T½ C18 mouse embryo fibroblasts cells, in a dominant lethal mutation test in male and female mice, and in micronucleus tests in mice and rats.
Itraconazole did not affect the fertility of male or female rats treated orally with dosage levels of up to 40 mg/kg/day (5x MRHD), even though parental toxicity was present at this dosage level. More severe signs of parental toxicity, including death, were present in the next higher dosage level, 160 mg/kg/day (20x MRHD).
Pregnancy: Teratogenic effects. Pregnancy Category C:
Itraconazole was found to cause a dose-related increase in maternal toxicity, embryotoxicity, and teratogenicity in rats at dosage levels of approximately 40 to 160 mg/kg/day (5 to 20x MRHD), and in mice at dosage levels of approximately 80 mg/kg/day (10x MRHD). Itraconazole has been shown to cross the placenta in a rat model. In rats, the teratogenicity consisted of major skeletal defects; in mice, it consisted of encephaloceles and/or macroglossia.
There are no studies in pregnant women. Itraconazole should be used for the treatment of systemic fungal infections in pregnancy only if the benefit outweighs the potential risk.
Itraconazole should not be administered for the treatment of onychomycosis to pregnant patients or to women contemplating pregnancy. Itraconazole should not be administered to women of childbearing potential for the treatment of onychomycosis unless they are using effective measures to prevent pregnancy and they begin therapy on the second or third day following the onset of menses. Effective contraception should be continued throughout itraconazole therapy and for 2 months following the end of treatment.
During post-marketing experience, cases of congenital abnormalities have been reported. (See ADVERSE REACTIONS: Post-marketing Experience.)
Nursing Mothers:
Itraconazole is excreted in human milk; therefore, the expected benefits of itraconazole therapy for the mother should be weighed against the potential risk from exposure of itraconazole to the infant. The U.S. Public Health Service Centers for Disease Control and Prevention advises HIV-infected women not to breast-feed to avoid potential transmission of HIV to uninfected infants.
Pediatric Use:
The efficacy and safety of itraconazole have not been established in pediatric patients.
The long-term effects of itraconazole on bone growth in children are unknown. In three toxicology studies using rats, itraconazole induced bone defects at dosage levels as low as 20 mg/kg/day (2.5x MRHD). The induced defects included reduced bone plate activity, thinning of the zona compacta of the large bones, and increased bone fragility. At a dosage level of 80 mg/kg/day (10x MRHD) over 1 year or 160 mg/kg/day (20x MRHD) for 6 months, itraconazole induced small tooth pulp with hypocellular appearance in some rats.
Geriatric Use:
Clinical studies of itraconazole capsules did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. It is advised to use itraconazole capsules in these patients only if it is determined that the potential benefit outweighs the potential risks. In general, it is recommended that the dose selection for an elderly patient should be taken into consideration, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Transient or permanent hearing loss has been reported in elderly patients receiving treatment with itraconazole. Several of these reports included concurrent administration of quinidine which is contraindicated (see BOXED WARNING: Drug Interactions, CONTRAINDICATIONS: Drug Interactions and PRECAUTIONS: Drug Interactions).
HIV-Infected Patients:
Because hypochlorhydria has been reported in HIV-infected individuals, the absorption of itraconazole in these patients may be decreased.
Renal Impairment:
Limited data are available on the use of oral itraconazole in patients with renal impairment. The exposure of itraconazole may be lower in some patients with renal impairment. Caution should be exercised when itraconazole is administered in this patient population and dose adjustment may be needed. (See CLINICAL PHARMACOLOGY: Special Populations and DOSAGE AND ADMINISTRATION.)
Hepatic Impairment:
Limited data are available on the use of oral itraconazole in patients with hepatic impairment. Caution should be exercised when this drug is administered in this patient population. It is recommended that patients with impaired hepatic function be carefully monitored when taking itraconazole. It is recommended that the prolonged elimination half-life of itraconazole observed in the single oral dose clinical trial with itraconazole capsules in cirrhotic patients be considered when deciding to initiate therapy with other medications metabolized by CYP3A4.
In patients with elevated or abnormal liver enzymes or active liver disease, or who have experienced liver toxicity with other drugs, treatment with itraconazole is strongly discouraged unless there is a serious or life-threatening situation where the expected benefit exceeds the risk. It is recommended that liver function monitoring be done in patients with preexisting hepatic function abnormalities or those who have experienced liver toxicity with other medications. (See CLINICAL PHARMACOLOGY: Special Populations and DOSAGE AND ADMINISTRATION.)
-
ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Itraconazole has been associated with rare cases of serious hepatotoxicity, including liver failure and death. Some of these cases had neither pre-existing liver disease nor a serious underlying medical condition. If clinical signs or symptoms develop that are consistent with liver disease, treatment should be discontinued and liver function testing performed. The risks and benefits of itraconazole use should be reassessed. (See WARNINGS: Hepatic Effects and PRECAUTIONS: Hepatotoxicity and Information for Patients.)
Adverse Events in the Treatment of Systemic Fungal Infections
Adverse event data were derived from 602 patients treated for systemic fungal disease in U.S. clinical trials who were immunocompromised or receiving multiple concomitant medications. Treatment was discontinued in 10.5% of patients due to adverse events. The median duration before discontinuation of therapy was 81 days (range: 2 to 776 days). The table lists adverse events reported by at least 1% of patients.
Table 2: Clinical Trials of Systemic Fungal Infections: Adverse Events Occurring with an Incidence of Greater than or Equal to 1%
Body System/Adverse Event
Incidence (%) (N=602)
Gastrointestinal
Nausea
Vomiting
Diarrhea
Abdominal Pain
Anorexia
11
5
3
2
1
Body as a Whole
Edema
Fatigue
Fever
Malaise
4
3
3
1
Skin and Appendages
Rash*
Pruritus
9
3
Central/Peripheral Nervous System
Headache
Dizziness
4
2
Psychiatric
Libido Decreased
Somnolence
1
1
Cardiovascular
Hypertension
3
Metabolic/Nutritional
Hypokalemia
2
Urinary System
Albuminuria
1
Liver and Biliary System
Hepatic Function Abnormal
3
Reproductive System, Male
Impotence
1
* Rash tends to occur more frequently in immunocompromised patients receiving immunosuppressive medications.
Adverse events infrequently reported in all studies included constipation, gastritis, depression, insomnia, tinnitus, menstrual disorder, adrenal insufficiency, gynecomastia, and male breast pain.
Adverse Events Reported in Toenail Onychomycosis Clinical Trials
Patients in these trials were on a continuous dosing regimen of 200 mg once daily for 12 consecutive weeks.
The following adverse events led to temporary or permanent discontinuation of therapy.
Table 3: Clinical Trials of Onychomycosis of the Toenail: Adverse Events Leading to Temporary or Permanent Discontinuation of Therapy
Adverse Event
Incidence (%)
Itraconazole (N=112)
Elevated Liver Enzymes (greater than twice the upper limit of normal)
4
Gastrointestinal Disorders
4
Rash
3
Hypertension
2
Orthostatic Hypotension
1
Headache
1
Malaise
1
Myalgia
1
Vasculitis
1
Vertigo
1
The following adverse events occurred with an incidence of greater than or equal to 1% (N=112): headache: 10%; rhinitis: 9%; upper respiratory tract infection: 8%; sinusitis, injury: 7%; diarrhea, dyspepsia, flatulence, abdominal pain, dizziness, rash: 4%; cystitis, urinary tract infection, liver function abnormality, myalgia, nausea: 3%; appetite increased, constipation, gastritis, gastroenteritis, pharyngitis, asthenia, fever, pain, tremor, herpes zoster, abnormal dreaming: 2%.
Adverse Events Reported in Fingernail Onychomycosis Clinical Trials
Patients in these trials were on a dosing regimen consisting of two 1-week treatment periods of 200 mg twice daily, separated by a 3-week period without drug.
The following adverse events led to temporary or permanent discontinuation of therapy.
Table 4: Clinical Trials of Onychomycosis of the Fingernail: Adverse Events Leading to Temporary or Permanent Discontinuation of Therapy
Adverse Event
Incidence (%)
Itraconazole (N=37)
Rash/Pruritus
3
Hypertriglyceridemia
3
The following adverse events occurred with an incidence of greater than or equal to 1% (N=37): headache: 8%; pruritus, nausea, rhinitis: 5%; rash, bursitis, anxiety, depression, constipation, abdominal pain, dyspepsia, ulcerative stomatitis, gingivitis, hypertriglyceridemia, sinusitis, fatigue, malaise, pain, injury: 3%.
Adverse Events Reported from Other Clinical Trials
In addition, the following adverse drug reaction was reported in patients who participated in itraconazole capsules clinical trials: Hepatobiliary Disorders: hyperbilirubinemia.
The following is a list of additional adverse drug reactions associated with itraconazole that have been reported in clinical trials of itraconazole Oral Solution and itraconazole IV excluding the adverse reaction term “Injection site inflammation” which is specific to the injection route of administration:
Cardiac Disorders: cardiac failure, left ventricular failure, tachycardia;
General Disorders and Administration Site Conditions: face edema, chest pain, chills;
Hepatobiliary Disorders: hepatic failure, jaundice;
Investigations: alanine aminotransferase increased, aspartate aminotransferase increased, blood alkaline phosphatase increased, blood lactate dehydrogenase increased, blood urea increased, gamma-glutamyltransferase increased, urine analysis abnormal;
Metabolism and Nutrition Disorders: hyperglycemia, hyperkalemia, hypomagnesemia;
Psychiatric Disorders: confusional state;
Renal and Urinary Disorders: renal impairment;
Respiratory, Thoracic and Mediastinal Disorders: dysphonia, cough;
Skin and Subcutaneous Tissue Disorders: rash erythematous, hyperhidrosis;
Vascular Disorders: hypotension
Post-marketing Experience
Adverse drug reactions that have been first identified during post-marketing experience with itraconazole(all formulations) are listed in the table below. Because these reactions are reported voluntarily from a population of uncertain size, reliably estimating their frequency or establishing a causal relationship to drug exposure is not always possible.
Table 5: Postmarketing Reports of Adverse Drug Reactions
Blood and Lymphatic System Disorders:
Leukopenia, neutropenia, thrombocytopenia
Immune System Disorders:
Anaphylaxis; anaphylactic, anaphylactoid and allergic reactions; serum sickness; angioneurotic edema
Nervous System Disorders:
Peripheral neuropathy, paresthesia, hypoesthesia, tremor
Eye Disorders:
Visual disturbances, including vision blurred and diplopia
Ear and Labyrinth Disorders:
Transient or permanent hearing loss
Cardiac Disorders:
Congestive heart failure
Respiratory, Thoracic and Mediastinal Disorders:
Pulmonary edema, dyspnea
Gastrointestinal Disorders:
Pancreatitis, dysgeusia
Hepatobiliary Disorders:
Serious hepatotoxicity (including some cases of fatal acute liver failure), hepatitis
Skin and Subcutaneous Tissue Disorders:
Toxic epidermal necrolysis, Stevens-Johnson syndrome, acute generalized exanthematous pustulosis, erythema multiforme, exfoliative dermatitis, leukocytoclastic vasculitis, alopecia, photosensitivity, urticaria
Musculoskeletal and Connective Tissue Disorders:
Arthralgia
Renal and Urinary Disorders:
Urinary incontinence, pollakiuria
Reproductive System and Breast Disorders:
Erectile dysfunction
General Disorders and Administration Site Conditions:
Peripheral edema
Investigations:
Blood creatine phosphokinase increased
There is limited information on the use of itraconazole during pregnancy. Cases of congenital abnormalities including skeletal, genitourinary tract, cardiovascular and ophthalmic malformations as well as chromosomal and multiple malformations have been reported during post-marketing experience. A causal relationship with itraconazole has not been established. See CLINICAL PHARMACOLOGY: Special Populations, CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS: Drug Interactions for more information.)
-
OVERDOSAGE
Itraconazole is not removed by dialysis. In the event of accidental overdosage, supportive measures should be employed. Activated charcoal may be given if considered appropriate. In general, adverse events reported with overdose have been consistent with adverse drug reactions already listed in this package insert for itraconazole. (See ADVERSE REACTIONS.)
-
DOSAGE AND ADMINISTRATION
Itraconazole capsulesshould be taken with a full meal to ensure maximal absorption. Itraconazole capsules must be swallowed whole.
Itraconazole capsule is a different preparation than itraconazole Oral Solution and should not be used interchangeably.
Treatment of Blastomycosis and Histoplasmosis:
The recommended dose is 200 mg once daily (2 capsules). If there is no obvious improvement, or there is evidence of progressive fungal disease, the dose should be increased in 100-mg increments to a maximum of 400 mg daily. Doses above 200 mg/day should be given in two divided doses.
Treatment of Aspergillosis:
A daily dose of 200 to 400 mg is recommended.
Treatment in Life-Threatening Situations:
In life-threatening situations, a loading dose should be used.
Although clinical studies did not provide for a loading dose, it is recommended, based on pharmacokinetic data, that a loading dose of 200 mg (2 capsules) three times daily (600 mg/day) be given for the first 3 days of treatment.
Treatment should be continued for a minimum of three months and until clinical parameters and laboratory tests indicate that the active fungal infection has subsided. An inadequate period of treatment may lead to recurrence of active infection.
Itraconazole capsules and itraconazole Oral Solution should not be used interchangeably. Only the oral solution has been demonstrated effective for oral and/or esophageal candidiasis.
Treatment of Onychomycosis:
Toenails with or without fingernail involvement: The recommended dose is 200 mg (2 capsules) once daily for 12 consecutive weeks.
Treatment of Onychomycosis:
Fingernails only: The recommended dosing regimen is two treatment courses, each consisting of 200 mg (2 capsules) b.i.d. (400 mg/day) for 1 week. The courses are separated by a 3-week period without itraconazole.
Use in Patients with Renal Impairment:
Limited data are available on the use of oral itraconazole in patients with renal impairment. Caution should be exercised when this drug is administered in this patient population. (See CLINICAL PHARMACOLOGY: Special Populations and PRECAUTIONS .)
Use in Patients with Hepatic Impairment:
Limited data are available on the use of oral itraconazole in patients with hepatic impairment. Caution should be exercised when this drug is administered in this patient population. (See CLINICAL PHARMACOLOGY: Special Populations, WARNINGS, and PRECAUTIONS.)
-
HOW SUPPLIED
Itraconazole capsules are supplied as follows:
100 mg:Blue opaque cap/pink transparent body size “0” hard gelatin capsules having imprinting “A” on cap with black ink and “175” on body with black ink filled with white to off-white pellets.
NDC 46708-204-30 Bottle of 30 Capsules
NDC 46708-204-90 Bottle of 90 Capsules
NDC 46708-204-71 Bottle of 500 Capsules
NDC 46708-204-10 Unit Dose Capsules of 100 (Blisters of 10)
NDC 46708-204-04 7 Blister Packs x 4 Capsules Each (7 Day Treatment Pack)Store at controlled room temperature 15° to 25°C (59° to 77°F). Protect from light and moisture.
Keep out of reach of children.
Manufactured by:
Alembic Pharmaceuticals Limited
Village Panelav, P. O. Tajpura, Near Baska,
Taluka-Halol, Panchmahal, Gujarat, India.
Revised: 09/2019
-
PATIENT INFORMATION LEAFLET
Itraconazole (IT-ra-KON-a-zole) Capsules
Rx OnlyThis summary contains important information about itraconazole.
This information is for patients who have been prescribed itraconazole to treat fungal nail infections. If your doctor prescribed itraconazole for medical problems other than fungal nail infections, ask your doctor if there is any information in this summary that does not apply to you. Read this information carefully each time you start to use itraconazole. This information does not take the place of discussion between you and your doctor. Only your doctor can decide if itraconazole is the right treatment for you. If you do not understand some of this information or have any questions, talk with your doctor or pharmacist.
WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT ITRACONAZOLE?
Itraconazole is used to treat fungal nail infections. However, itraconazole is not for everyone. Do not take itraconazole for fungal nail infections if you have had heart failure, including congestive heart failure. You should not take itraconazole if you are taking certain medicines that could lead to serious or life-threatening medical problems.
(See “Who Should Not Take Itraconazole?” below.)
If you have had heart, lung, liver, kidney or other serious health problems, ask your doctor if it is safe for you to take itraconazole.
WHAT HAPPENS IF I HAVE A FUNGAL NAIL INFECTION?
Anyone can have a fungal nail infection, but it is usually found in adults. When a fungus infects the tip or sides of a nail, the infected part of the nail may turn yellow or brown. If not treated, the fungus may spread under the nail towards the cuticle. If the fungus spreads, more of the nail may change color, may become thick or brittle, and the tip of the nail may become raised. In some patients, this can cause pain and discomfort.
WHAT IS ITRACONAZOLE?
Itraconazole is a prescription medicine used to treat fungal infections of the toenails and fingernails. It is also used to treat some types of fungal infections in other areas of your body. We do not know if itraconazole works in children with fungal nail infections or if it is safe for children to take.
Itraconazole comes in the form of capsules and liquid (oral solution). The capsule and liquid forms work differently, so you should not use one in place of the other. This Patient Information discusses only the capsule form of itraconazole. You will get these capsules in a medicine bottle.
Itraconazole goes into your bloodstream and travels to the source of the infection underneath the nail so that it can fight the infection there. Improved nails may not be obvious for several months after the treatment period is finished because it usually takes about 6 months to grow a new fingernail and 12 months to grow a new toenail.
WHO SHOULD NOT TAKE ITRACONAZOLE?
Itraconazole are not for everyone. Your doctor will decide if itraconazole is the right treatment for you. Some patients should not take itraconazole because they may have certain health problems or may be taking certain medicines that could lead to serious or life-threatening medical problems.
Tell your doctor and pharmacist the name of all the prescription and non-prescription medicines you are taking, including dietary supplements and herbal remedies. Also tell your doctor about any other medical conditions you have had, especially heart, lung, liver or kidney conditions; or if you have cystic fibrosis, or have had an allergic reaction to itraconazole or any other antifungal medicines.
Never take itraconazole if you:
- have had heart failure, including congestive heart failure.
- are taking any of the medicines listed below. Dangerous or even life-threatening side effects could result:
•cisapride (such as Propulsid®)
•colchicine (such as Colcrys™) [if you also have pre-existing kidney or liver impairment]
•disopyramide (such as Norpace®)
•dofetilide (such as Tikosyn™)
•dronedarone (such as Multaq®)
•eplerenone (such as Inspra®)
•ergot alkaloids (such as Migranal®, Ergonovine, Cafergot®, Methergine®)
•felodipine (such as Plendil®)
•fesoterodine (such as Toviaz®) [if you also have pre-existing kidney or liver impairment]
•irinotecan (such as Camptosar®)
•lovastatin (such as Mevacor®, Advicor®, Altocor™)
•lurasidone (such as Latuda®)
•methadone (such as Dolophine®)
•midazolam (such as Versed®)
•nisoldipine (such as Sular®)
•pimozide (such as Orap®)
•quinidine (such as Cardioquin®, Quinaglute®, Quinidex®)
•ranolazine (such as Ranexa®)
•simvastatin (such as Zocor®)
•solifenacin (such as Vesicare®) [if you also have pre-existing kidney or liver impairment]
•telithromycin (such as Ketek®) [if you also have pre-existing kidney or liver impairment]
•ticagrelor (such as Brilinta®)
•triazolam (such as Hacion®)
· have ever had an allergic reaction to itraconazole
Taking itraconazole with certain other medicines may lead to serious or life-threatening medical problems. Tell your doctor and pharmacist the name of all the prescription and non-prescription medicines you are taking, including dietary supplements and herbal remedies. . Your doctor will decide if itraconazole is the right treatment for you.
WHAT SHOULD I KNOW ABOUT ITRACONAZOLE AND PREGNANCY OR BREAST FEEDING?
Never take itraconazole if you have a fungal nail infection and are pregnant or planning to become pregnant within 2 months after you have finished your treatment.
If you are able to become pregnant, you should use effective birth control during itraconazole treatment and for 2 months after finishing treatment. Ask your doctor about effective types of birth control.
If you are breast-feeding, talk with your doctor about whether you should take itraconazole.
HOW SHOULD I TAKE ITRACONAZOLE CAPSULES?
Always take itraconazole capsules during or right after a full meal.
Your doctor will decide the right dose for you. Depending on your infection, you will take itraconazole once a day for 12 weeks, or twice a day for 1 week in a dosing schedule. You will receive a bottle of capsules. Do not skip any doses. Be sure to finish all your itraconazole as prescribed by your doctor.
If you have ever had liver problems, your doctor should do a blood test to check your condition. If you haven’t had liver problems, your doctor may recommend blood tests to check the condition of your liver because patients taking itraconazole can develop liver problems.
Itraconazole can sometimes cause dizziness or blurred/double vision. If you have these symptoms, do not drive or use machines.If you forget to take or miss doses of itraconazole, ask your doctor what you should do with the missed doses.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF ITRACONAZOLE?
The most common side effects include: headache, and digestive system problems (such as nausea, and abdominal pain).
Stop itraconazole and call your doctor or get medical assistance right away if you have a severe allergic reaction. Symptoms of an allergic reaction may include skin rash, itching, hives, shortness of breath or difficulty breathing, and/or swelling of the face. Very rarely, an oversensitivity to sunlight, a tingling sensation in the limbs or a severe skin disorder can occur. If any of these symptoms occur, stop taking itraconazole and contact your doctor.
Stop itraconazole and call your doctor right away if you develop shortness of breath; have unusual swelling of your feet, ankles or legs; suddenly gain weight; are unusually tired; cough up white or pink phlegm; have unusual fast heartbeats; or begin to wake up at night. In rare cases, patients taking itraconazole could develop serious heart problems, and these could be warning signs of heart failure.
Stop itraconazole and call your doctor right away if you become unusually tired; lose your appetite; or develop nausea, abdominal pain, or vomiting, a yellow color to your skin or eyes, or dark colored urine or pale stools (bowel movements). In rare cases, patients taking itraconazole could develop serious liver problems and these could be warning signs.
Stop itraconazole and call your doctor right away if you experience any hearing loss symptoms. In very rare cases, patients taking itraconazole have reported temporary or permanent hearing loss.
Call your doctor right away if you develop tingling or numbness in your extremities (hands or feet), if your vision gets blurry or you see double, if you hear a ringing in your ears, if you lose the ability to control your urine or urinate much more than usual.
Additional possible side effects include upset stomach, vomiting, constipation, fever, inflammation of the pancreas, menstrual disorder, erectile dysfunction, dizziness, muscle pain, painful joints, unpleasant taste, or hair loss. These are not all the side effects of itraconazole. Your doctor or pharmacist can give you a more complete list.
WHAT SHOULD I DO IF I TAKE AN OVERDOSE OF ITRACONAZOLE?
If you think you took too much itraconazole, call your doctor or local poison control center, or go to the nearest hospital emergency room right away.
HOW SHOULD I STORE ITRACONAZOLE?
Keep all medicines, including itraconazole, out of the reach of children.
Store itraconazole capsules at room temperature in a dry place away from light.
GENERAL ADVICE ABOUT ITRACONAZOLE
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use itraconazole for a condition for which it was not prescribed. Do not give itraconazole to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about itraconazole. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about itraconazole that is written for health professionals.
Call your doctor for medical advice about side effects. You may report side effects to Alembic Pharmaceuticals Limited at 1-866 210 9797 or FDA at 1-800-FDA-1088.
This patient information has been approvedby the U.S. Food and Drug Administration.
The brands listed are the registered trademarks of their respective owners, and are not trademarks of Alembic Pharmaceuticals Ltd.
Manufactured by:
Alembic Pharmaceuticals Limited
Village Panelav, P. O. Tajpura, Near Baska,Taluka-Halol, Panchmahal, Gujarat, India.
Revised: 09/2019
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ITRACONAZOLE
itraconazole capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:46708-204 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ITRACONAZOLE (UNII: 304NUG5GF4) (ITRACONAZOLE - UNII:304NUG5GF4) ITRACONAZOLE 100 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL 20000 (UNII: 5WKN5KL2O8) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 22 (UNII: 1678RKX8RT) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 28 (UNII: 767IP0Y5NH) GELATIN (UNII: 2G86QN327L) STARCH, CORN (UNII: O8232NY3SJ) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color BLUE (blue opaque pink transparent) Score no score Shape CAPSULE Size 21mm Flavor Imprint Code A;175 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46708-204-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2016 2 NDC:46708-204-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2016 3 NDC:46708-204-71 500 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2016 4 NDC:46708-204-10 100 in 1 CARTON; Type 0: Not a Combination Product 12/16/2016 5 NDC:46708-204-04 28 in 1 CARTON; Type 0: Not a Combination Product 12/16/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206741 12/16/2016 Labeler - Alembic Pharmaceuticals Limited (650574663) Establishment Name Address ID/FEI Business Operations Alembic Pharmaceuticals Limited 650574671 MANUFACTURE(46708-204)


