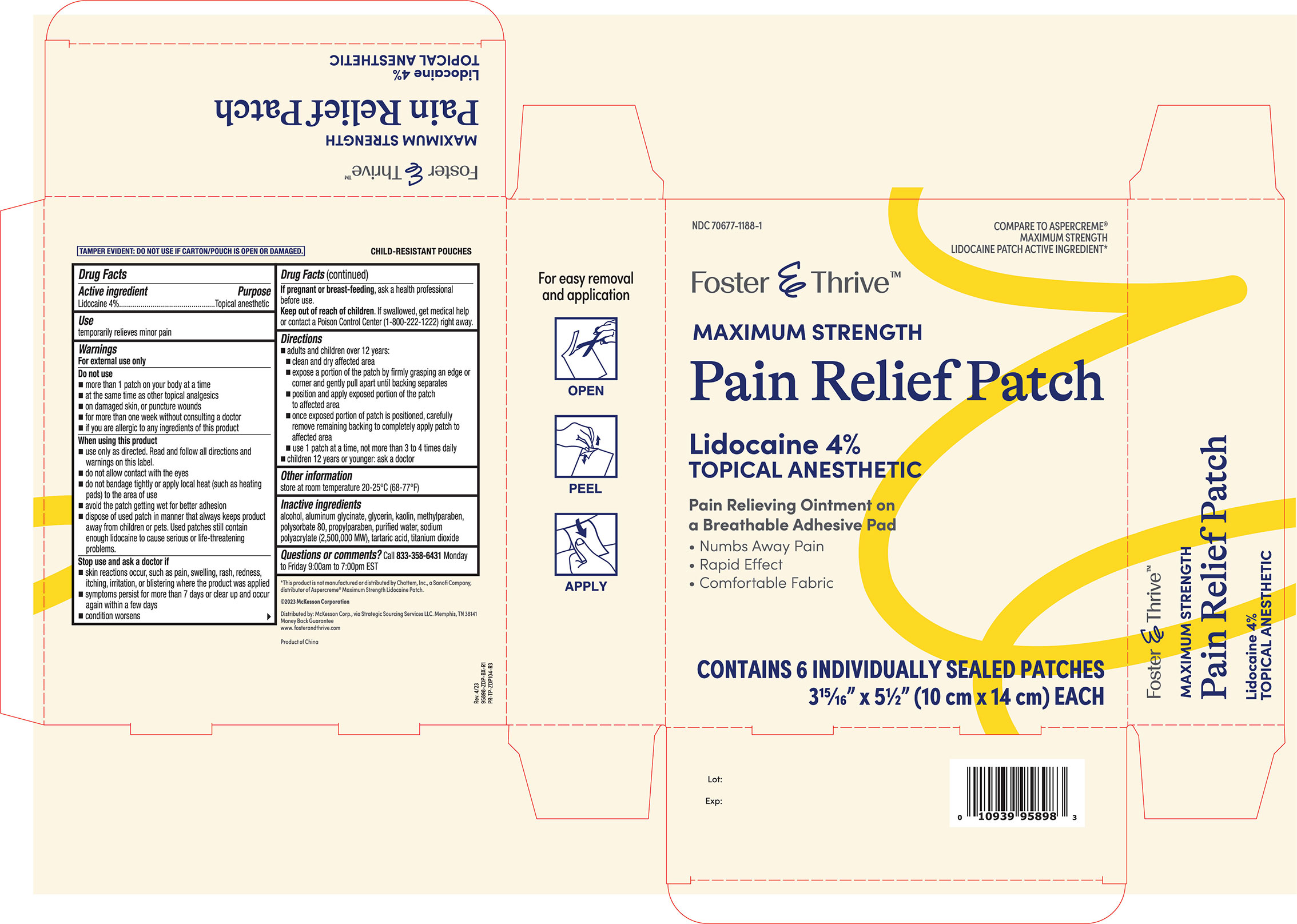
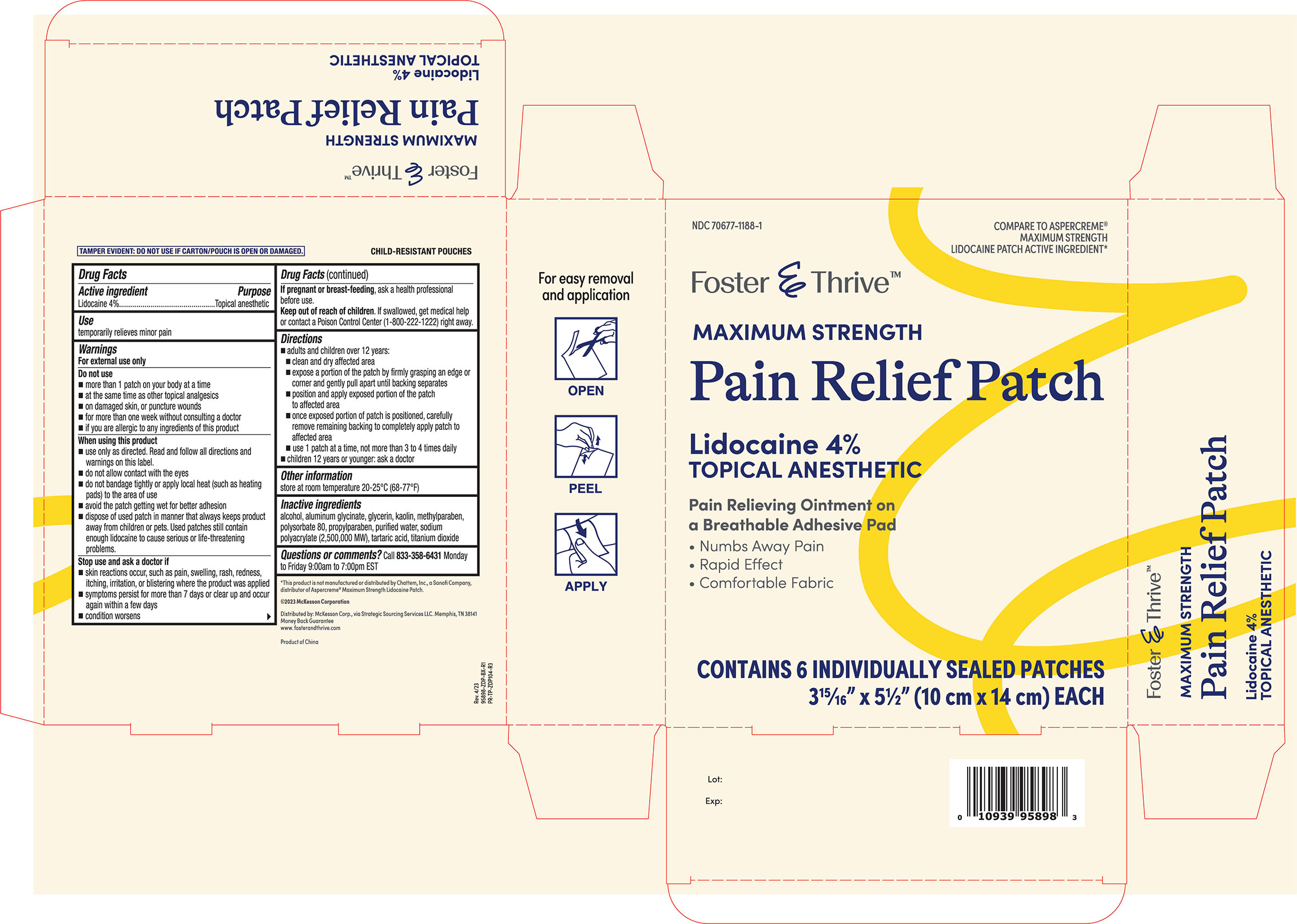
Label: FOSTER AND THRIVE- lidocaine patch
- NDC Code(s): 70677-1188-1
- Packager: STRATEGIC SOURCING SERVICES, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
-
WHEN USING
When using this product
- use only as directed. Read and follow all directions and warnings on this label.
- do not allow contact with the eyes
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
- avoid the patch getting wet for better adhesion
- dispose of used patch in manner that always keeps product away from children or pets. Used patches still contain enough lidocaine to cause serious or life-threating problems.
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Directions
- adults and children over 12 years:
- clean and dry affected area
- expose a portion of the patch by firmly grasping an edge or corner and gently pull apart until backing separates
- position and apply exposed portion of the patch to affected area
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- use 1 patch at a time, not more than 3 to 4 times daily
- children under 12 years or younger: ask a doctor
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOSTER AND THRIVE
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70677-1188 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength TARTARIC ACID (UNII: W4888I119H) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) ALCOHOL (UNII: 3K9958V90M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLYCERIN (UNII: PDC6A3C0OX) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) KAOLIN (UNII: 24H4NWX5CO) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70677-1188-1 6 in 1 CARTON 09/01/2023 1 1 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/01/2023 Labeler - STRATEGIC SOURCING SERVICES, LLC (116956644)