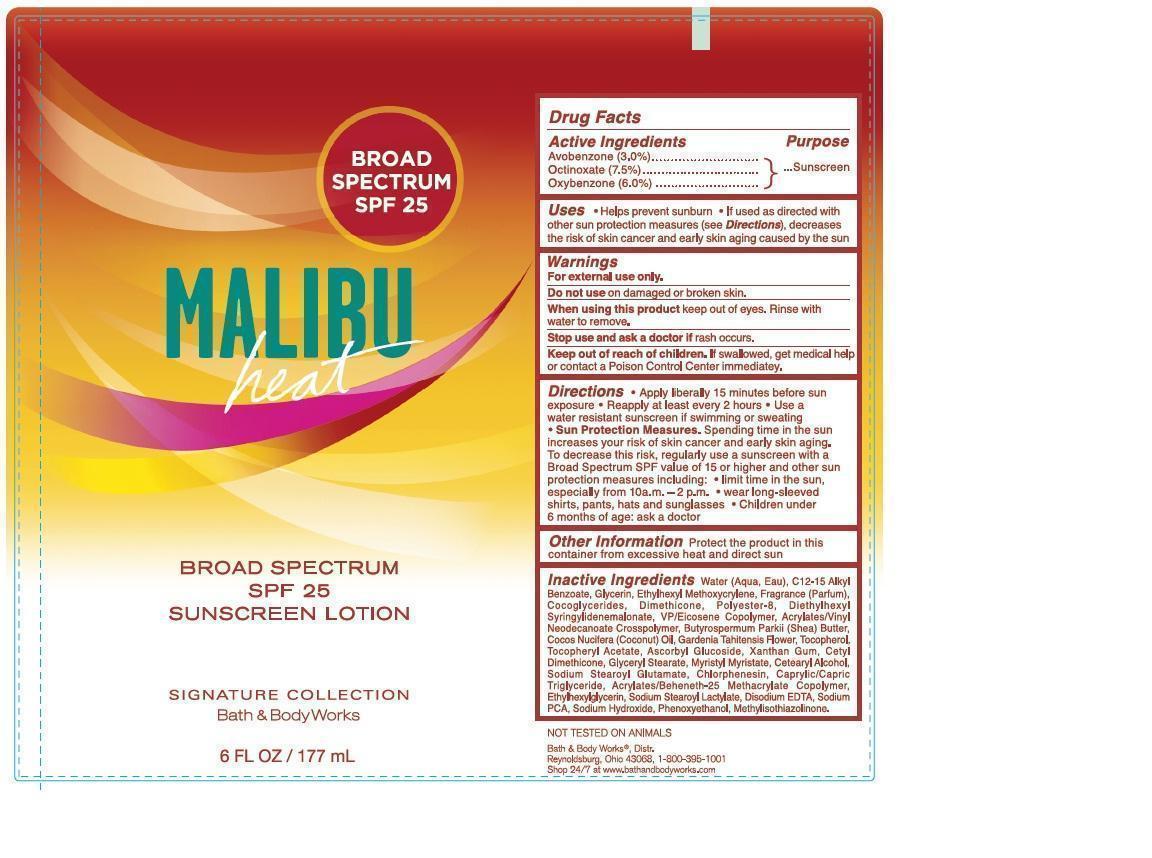
Label: SIGNATURE SUNSCREEN BROAD SPECTRUM SPF 25 MALIBU HEAT- avobenzone, octinoxate, oxybenzone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 62670-8045-5 - Packager: Bath & Body Works, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 9, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- DO NOT USE
- WHEN USING THIS PRODUCT
- STOP USE AND ASK A DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN.
-
DIRECTIONS
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
Water (Aqua, Eau), C12-15 Alkyl Benzoate, Glycerin, Ethylhexyl Methoxycrylene, Fragrance (Parfum), Cocoglycerides, Dimethicone, Polyester-8, Diethylhexyl Syringylidenemalonate, VP/Eicosene Copolymer, Acrylates/Vinyl Neodecanoate Crosspolymer, Butyrospermum Parkii (Shea) Butter, Cocos Nucifera (Coconut) Oil, Gardenia Tahitensis Flower, Tocopherol, Tocopheryl Acetate, Ascorbyl Glucoside, Xanthan Gum, Cetyl Dimethicone, Glyceryl Stearate, Myristyl Myristate, Cetearyl Alcohol, Sodium Stearoyl Glutamate, Chlorphenesin, Caprylic/Capric Triglyceride, Acrylates/Beheneth-25 Methacrylate Copolymer, Ethylhexylglycerin, Sodium Stearoyl Lactylate, Disodium EDTA, Sodium PCA, Sodium Hydroxide, Phenoxyethanol, Methylisothiazolinone.
- COMPANY INFORMATION
- PRODUCT PACKAGING
-
INGREDIENTS AND APPEARANCE
SIGNATURE SUNSCREEN BROAD SPECTRUM SPF 25 MALIBU HEAT
avobenzone, octinoxate, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62670-8045 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3.0 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 6.0 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62670-8045-5 177 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/09/2013 Labeler - Bath & Body Works, Inc. (878952845) Establishment Name Address ID/FEI Business Operations Cosmetic Labs of America 013696501 manufacture(62670-8045)