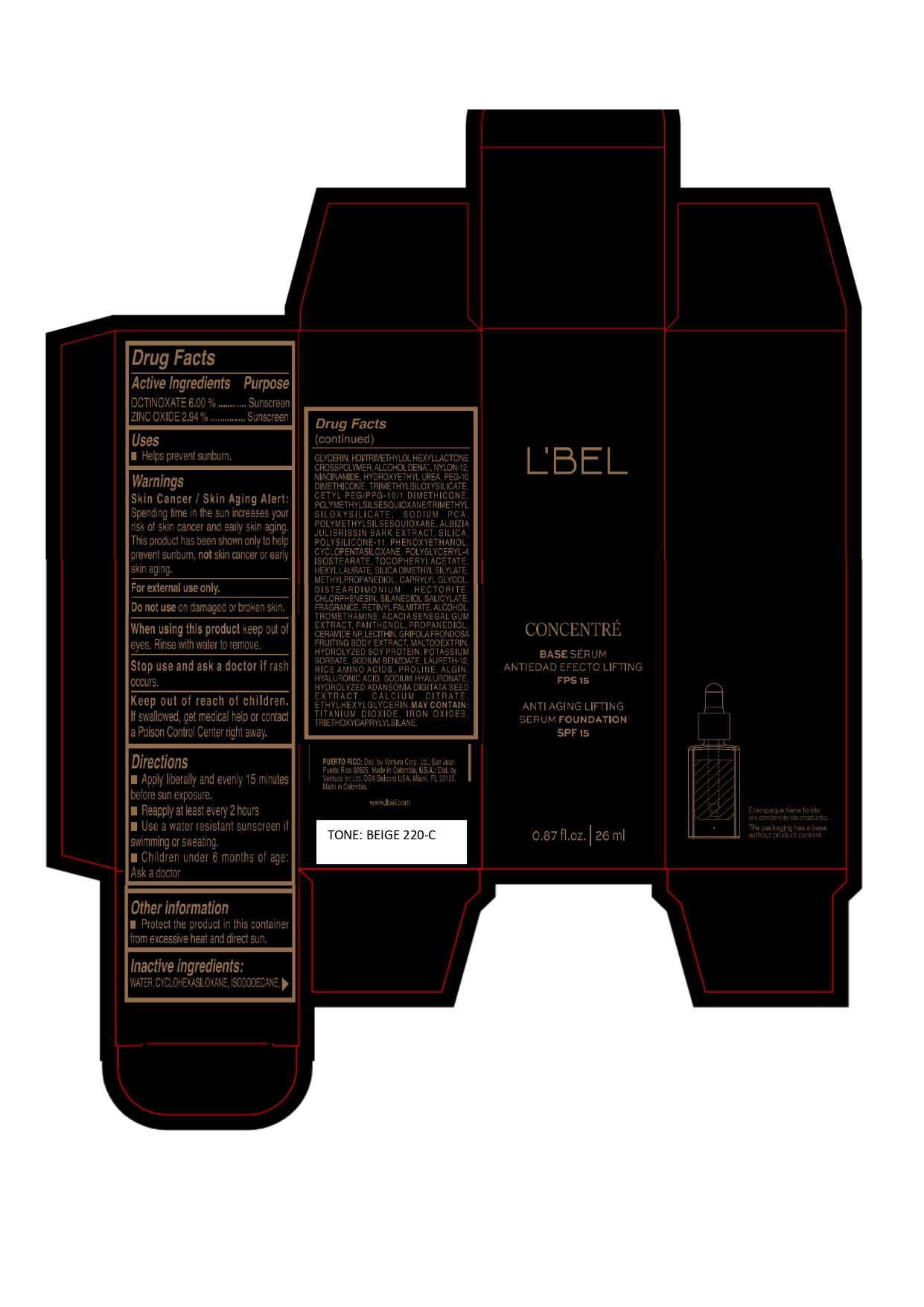
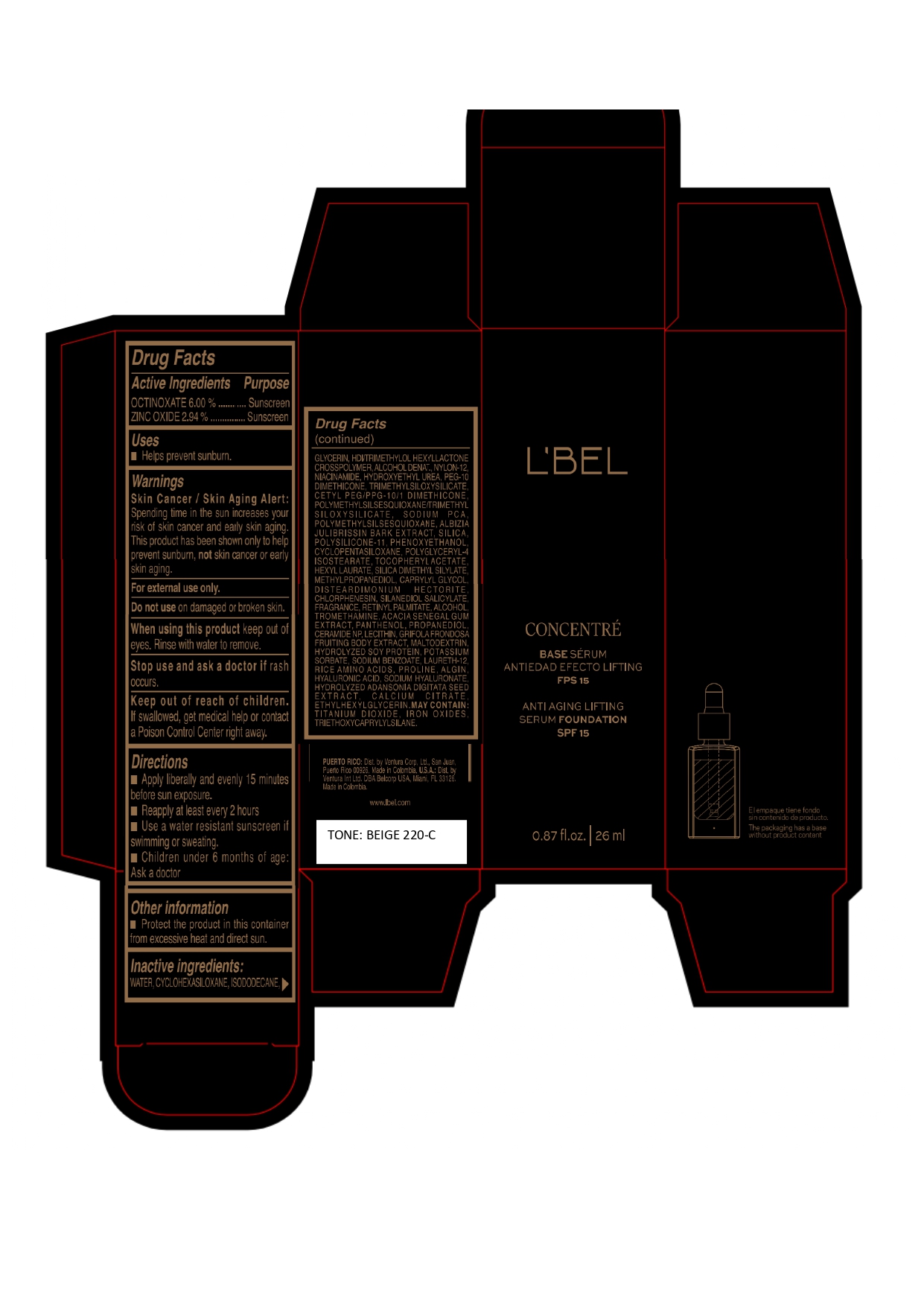
Label: LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 BEIGE 220-C- octinoxate, zinc oxide emulsion
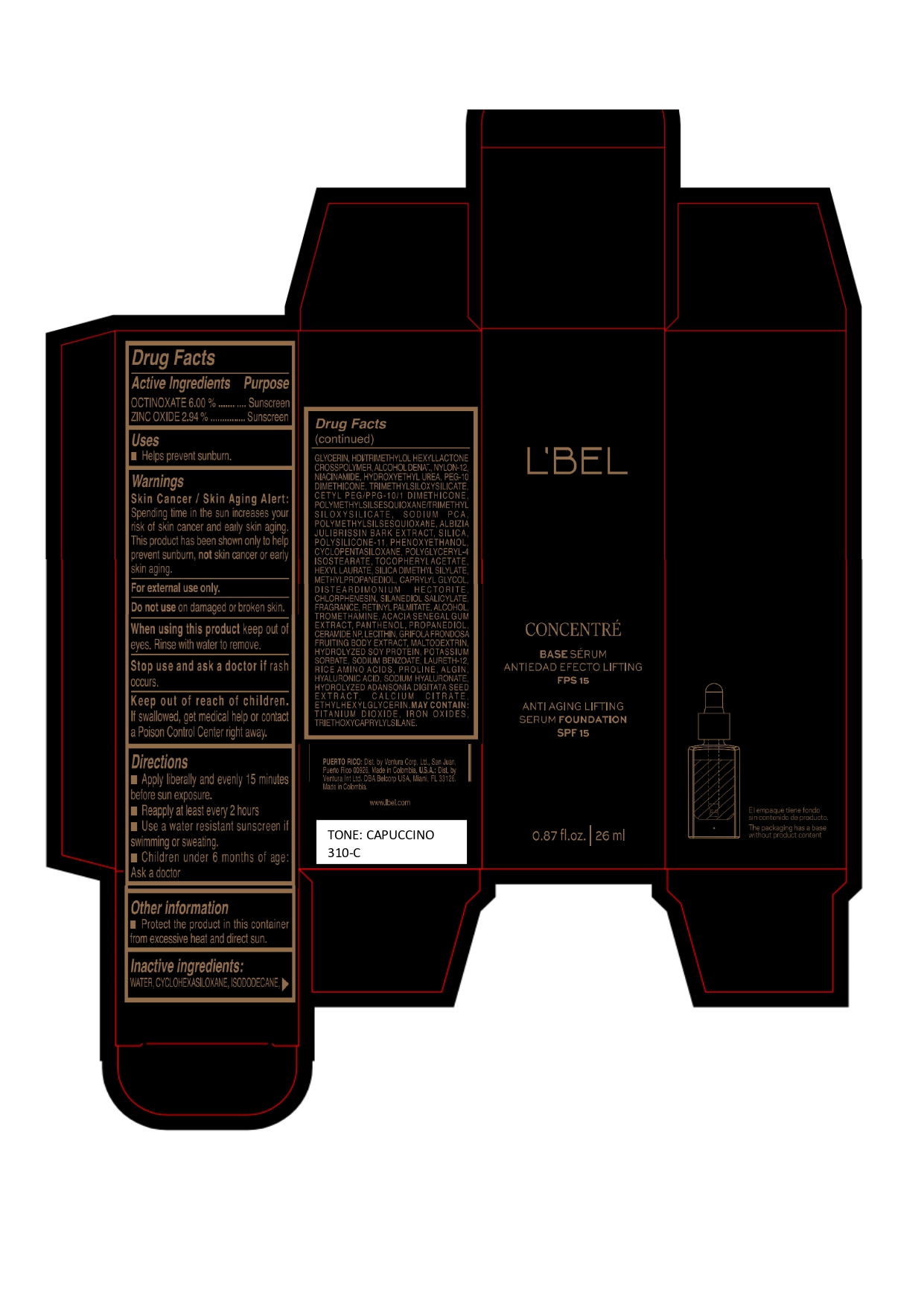
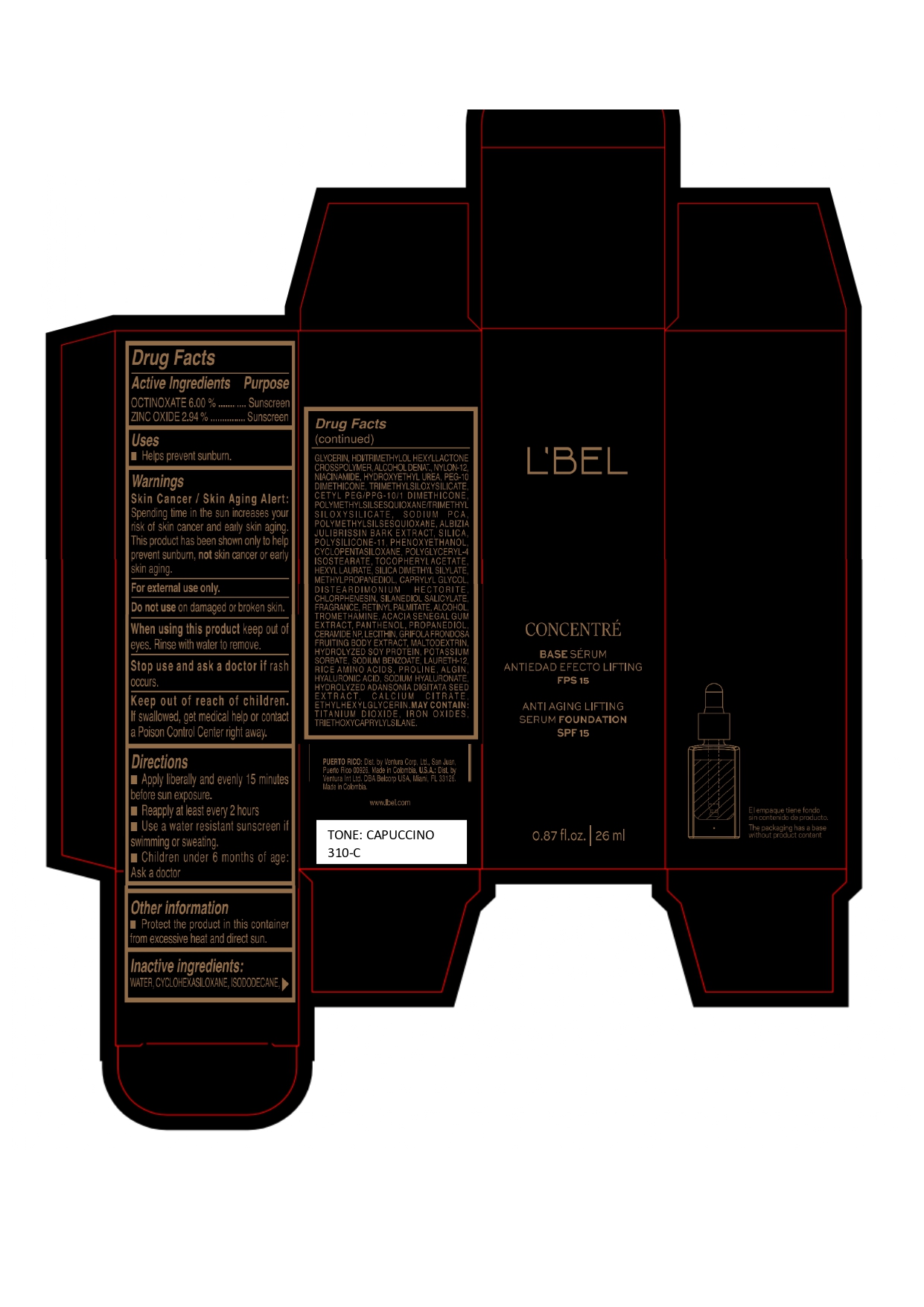
LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 CAPUCCINO 310-C- octinoxate, zinc oxide emulsion
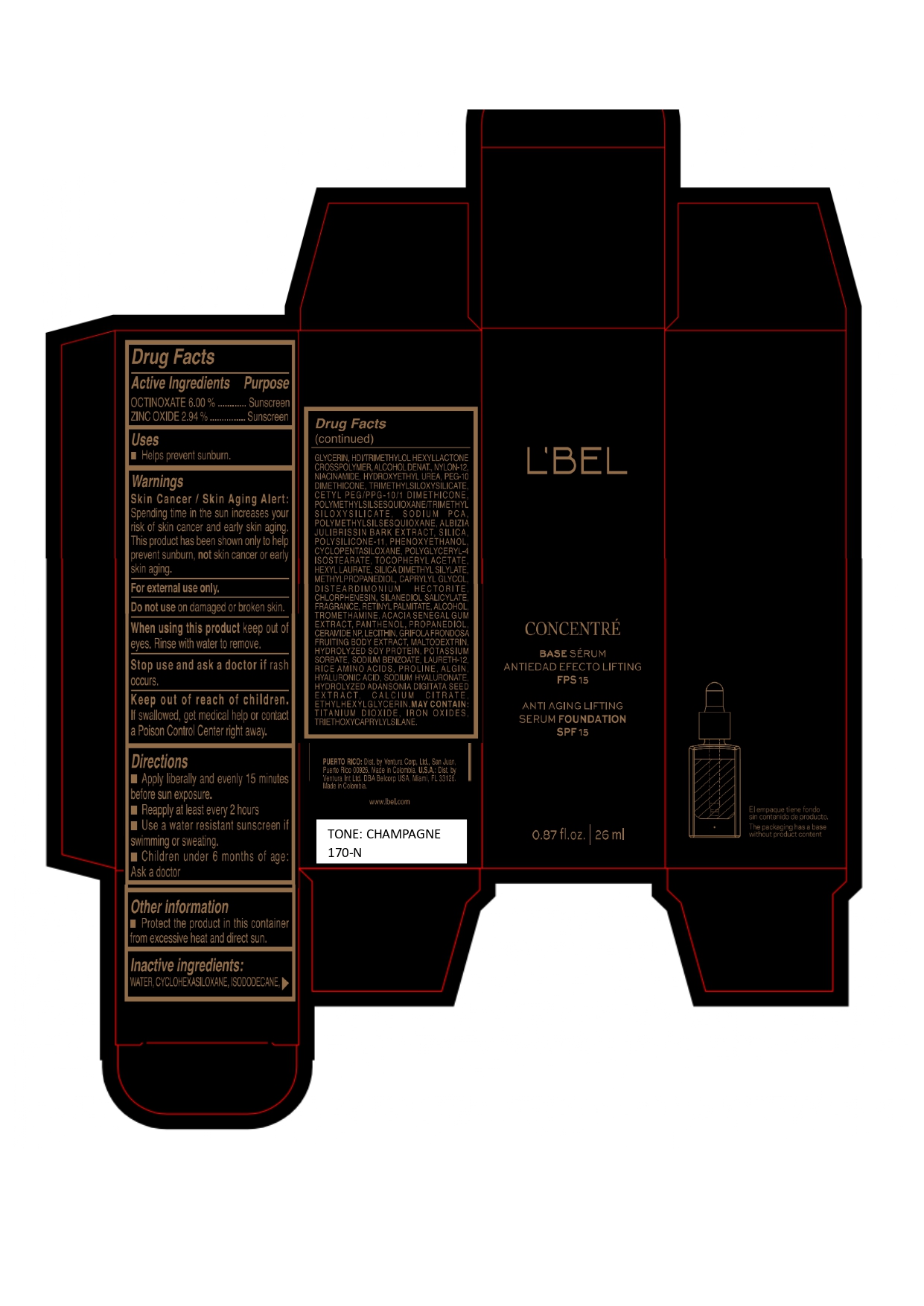
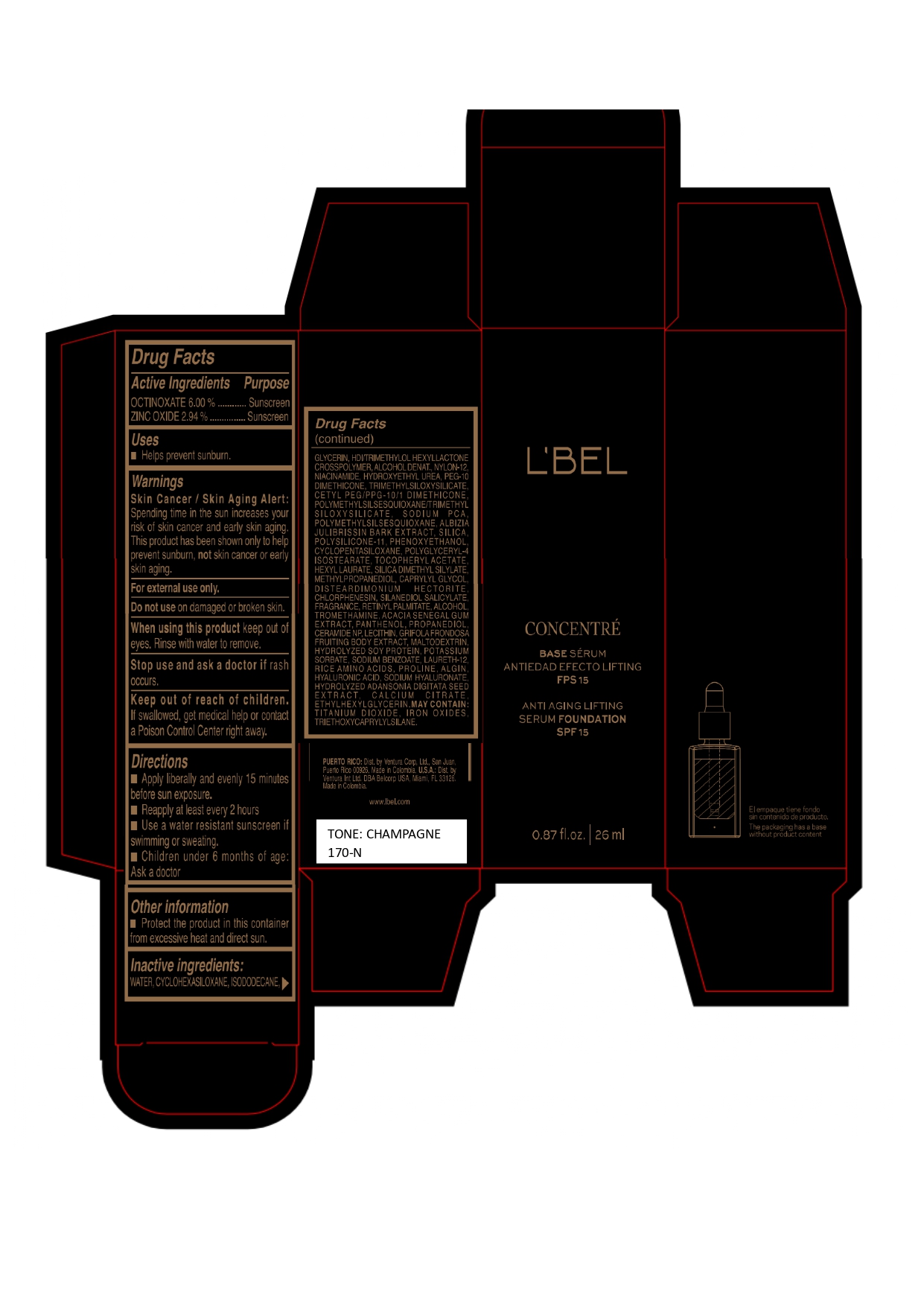
LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 CHAMPAGNE 170-N- octinoxate, zinc oxide emulsion
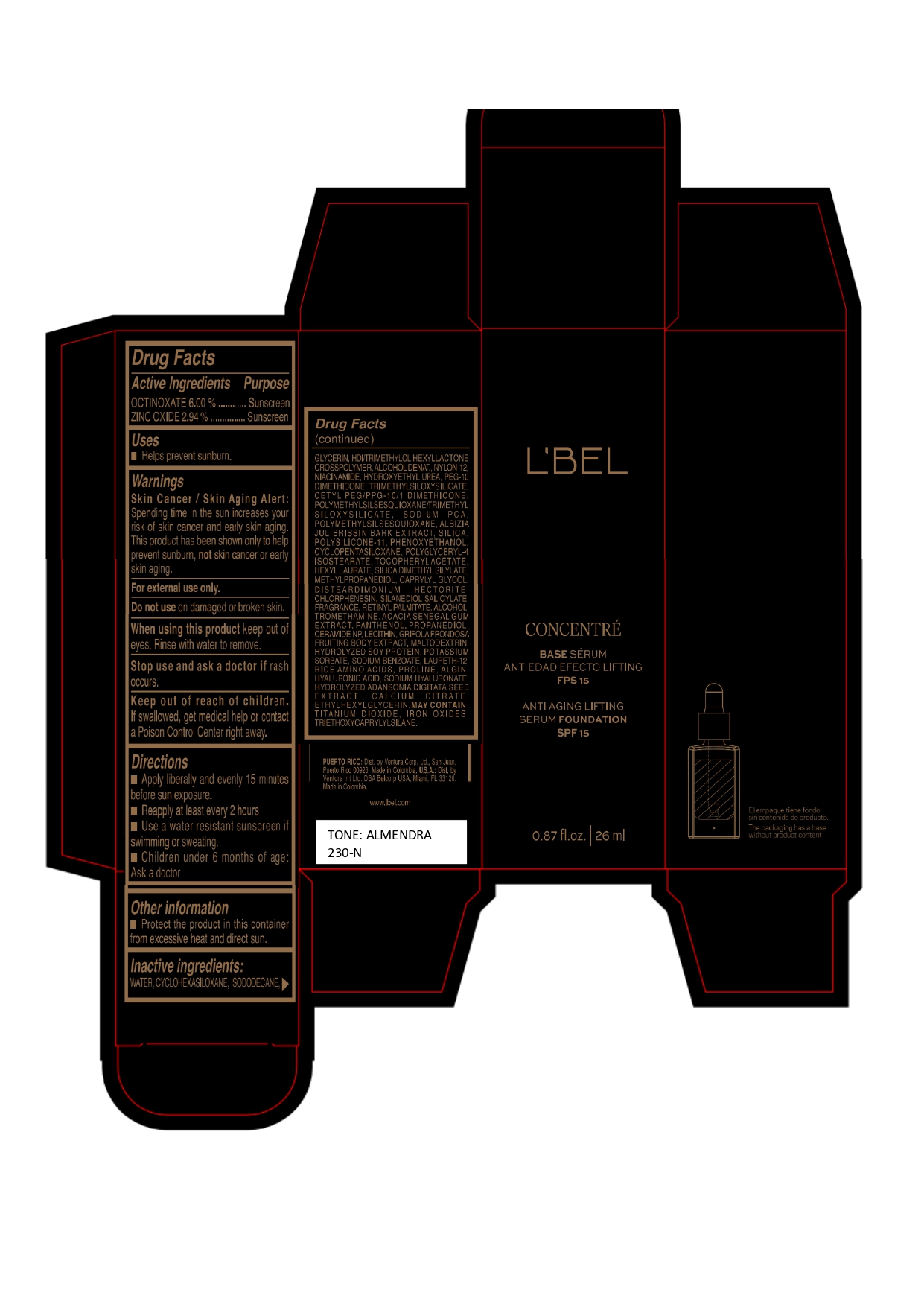
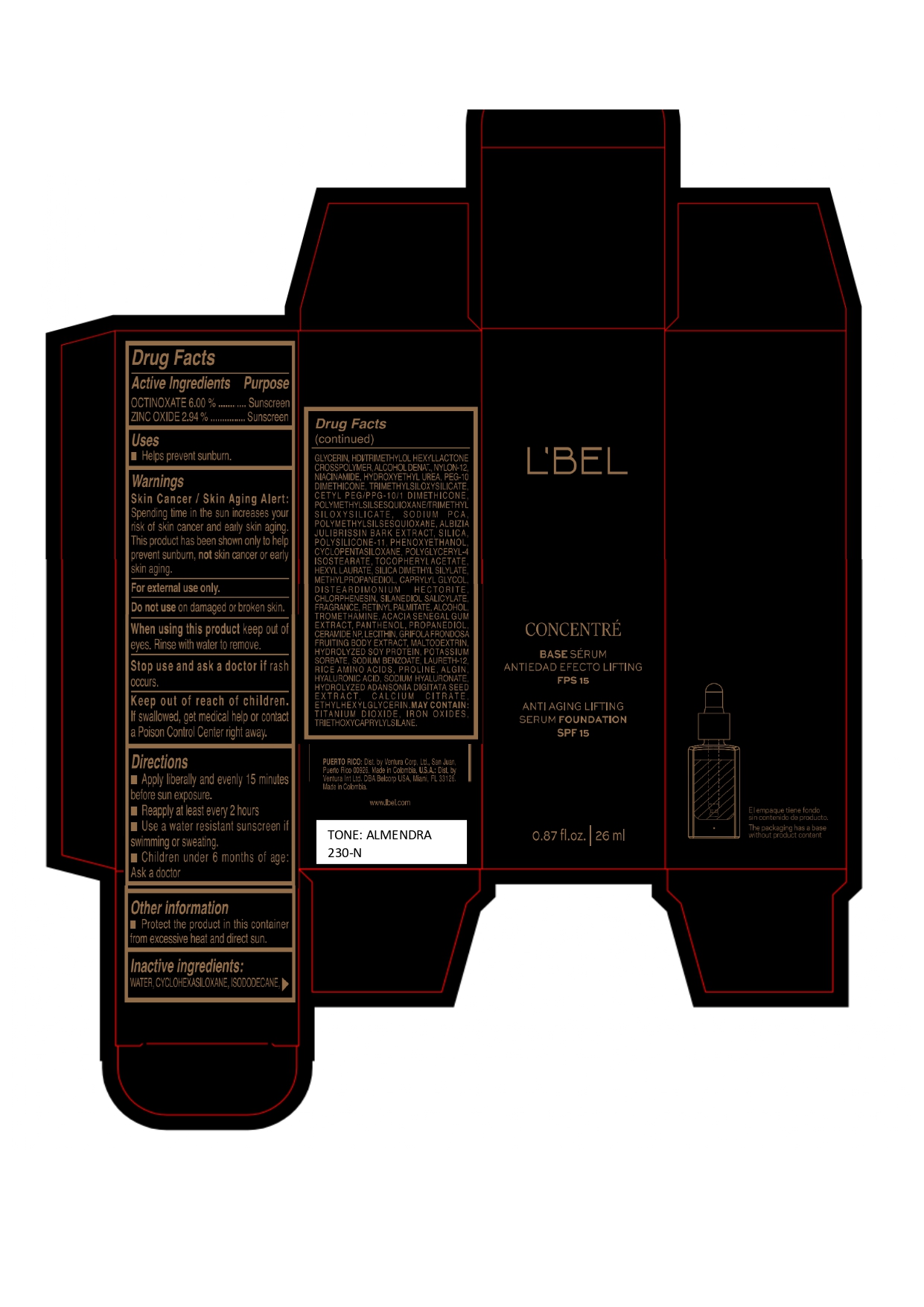
LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 ALMENDRA 230-N- octinoxate, zinc oxide emulsion
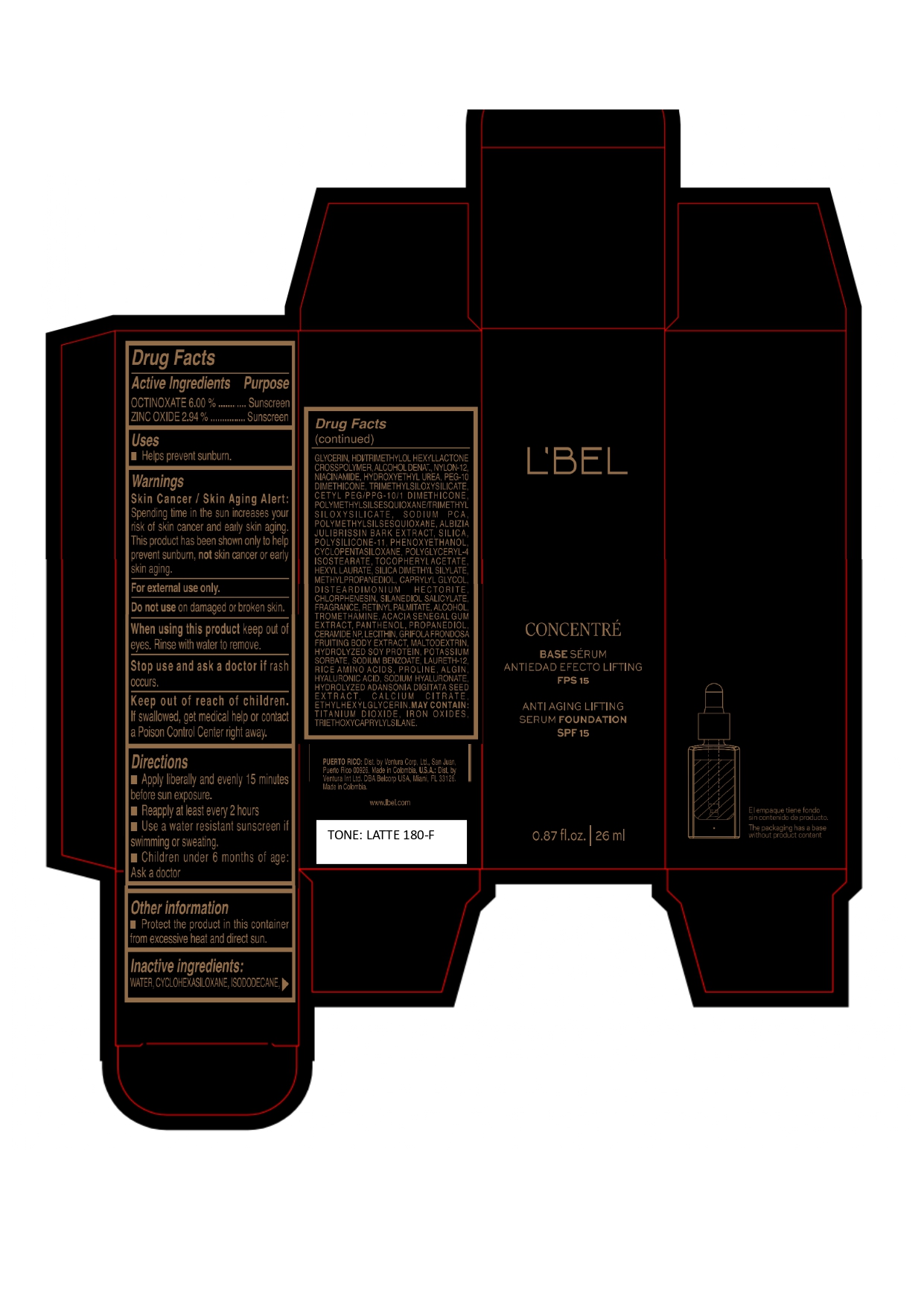
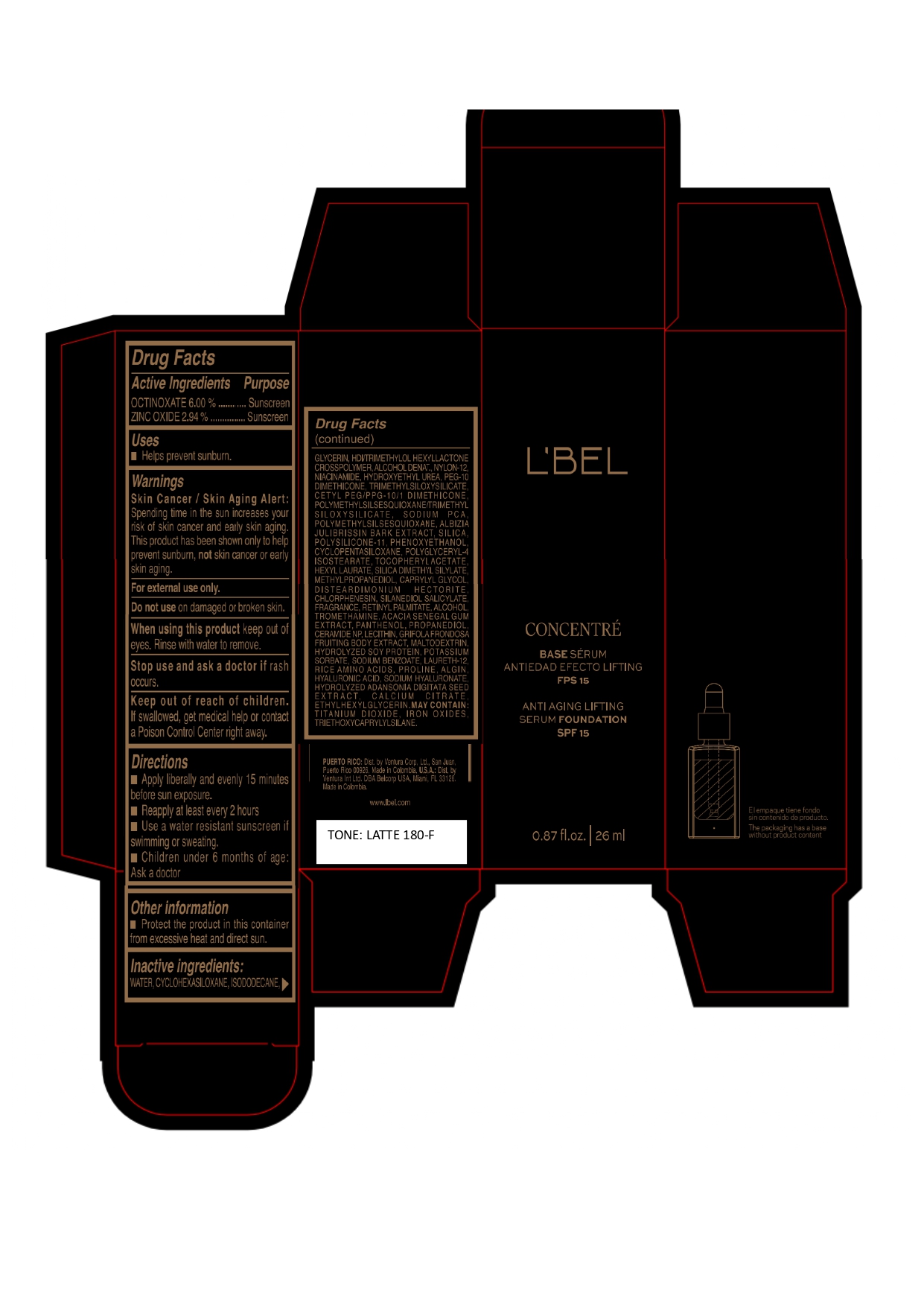
LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 LATTE 180-F- octinoxate, zinc oxide emulsion
-
NDC Code(s):
14141-326-01,
14141-327-01,
14141-328-01,
14141-329-01, view more14141-330-01
- Packager: BEL STAR SA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
WATER, CYCLOHEXASILOXANE, ISODODECANE, GLYCERIN, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, ALCOHOL DENAT., NYLON-12, NIACINAMIDE, HYDROXYETHYL UREA, PEG-10 DIMETHICONE, TRIMETHYLSILOXYSILICATE, CETYL PEG/PPG-10/1 DIMETHICONE, POLYMETHYLSILSESQUIOXANE/TRIMETHYLSILOXYSILICATE, SODIUM PCA, POLYMETHYLSILSESQUIOXANE, ALBIZIA JULIBRISSIN BARK EXTRACT, SILICA, POLYSILICONE-11, PHENOXYETHANOL, CYCLOPENTASILOXANE, POLYGLYCERYL-4 ISOSTEARATE, TOCOPHERYL ACETATE, HEXYL LAURATE, SILICA DIMETHYL SILYLATE, METHYLPROPANEDIOL, CAPRYLYL GLYCOL, DISTEARDIMONIUM HECTORITE, CHLORPHENESIN, SILANEDIOL SALICYLATE, FRAGRANCE, RETINYL PALMITATE, ALCOHOL, TROMETHAMINE, ACACIA SENEGAL GUM EXTRACT, PANTHENOL, PROPANEDIOL, CERAMIDE NP, LECITHIN, GRIFOLA FRONDOSA FRUITING BODY EXTRACT, MALTODEXTRIN, HYDROLYZED SOY PROTEIN, POTASSIUM SORBATE, SODIUM BENZOATE, LAURETH-12, RICE AMINO ACIDS, PROLINE, ALGIN, HYALURONIC ACID, SODIUM HYALURONATE, HYDROLYZED ADANSONIA DIGITATA SEED EXTRACT, CALCIUM CITRATE, ETHYLHEXYLGLYCERIN.
MAY CONTAIN :
TITANIUM DIOXIDE, IRON OXIDES, TRIETHOXYCAPRYLYLSILANE. - Company Information
- Package Label CHAMPAGNE 170-N
- Package Label LATTE 180-F
- Package Label BEIGE 220-C
- Package Label ALMENDRA 230-N
- Package Label CAPUCCINO 310-C
-
INGREDIENTS AND APPEARANCE
LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 BEIGE 220-C
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-328 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ISODODECANE (UNII: A8289P68Y2) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) CHLORPHENESIN (UNII: I670DAL4SZ) NYLON-12 (UNII: 446U8J075B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERRIC OXIDE RED (UNII: 1K09F3G675) GLYCERIN (UNII: PDC6A3C0OX) NIACINAMIDE (UNII: 25X51I8RD4) MALTODEXTRIN (UNII: 7CVR7L4A2D) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PEG-10 DIMETHICONE (220 CST) (UNII: 287GF3Y3WC) POLYMETHYLSILSESQUIOXANE/TRIMETHYLSILOXYSILICATE (UNII: X2PZH4Y6HT) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYLPROPANEDIOL (UNII: N8F53B3R4R) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SILANEDIOL SALICYLATE (UNII: C054DF30K0) TROMETHAMINE (UNII: 023C2WHX2V) CERAMIDE NP (UNII: 4370DF050B) SODIUM BENZOATE (UNII: OJ245FE5EU) PROLINE (UNII: 9DLQ4CIU6V) CALCIUM CITRATE (UNII: MLM29U2X85) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) MAITAKE (UNII: A1U5YJI0Z8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LAURETH-12 (UNII: OAH19558U1) SODIUM ALGINATE (UNII: C269C4G2ZQ) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) AMINO ACIDS, RICE (UNII: 5ET1T25H82) ADANSONIA DIGITATA SEED (UNII: 2936P60TPX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PANTHENOL (UNII: WV9CM0O67Z) HYALURONIC ACID (UNII: S270N0TRQY) ACACIA (UNII: 5C5403N26O) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-328-01 1 in 1 BOX 04/19/2023 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 04/19/2023 LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 CAPUCCINO 310-C
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-330 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ISODODECANE (UNII: A8289P68Y2) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) CHLORPHENESIN (UNII: I670DAL4SZ) NYLON-12 (UNII: 446U8J075B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERRIC OXIDE RED (UNII: 1K09F3G675) GLYCERIN (UNII: PDC6A3C0OX) NIACINAMIDE (UNII: 25X51I8RD4) MALTODEXTRIN (UNII: 7CVR7L4A2D) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PEG-10 DIMETHICONE (220 CST) (UNII: 287GF3Y3WC) POLYMETHYLSILSESQUIOXANE/TRIMETHYLSILOXYSILICATE (UNII: X2PZH4Y6HT) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYLPROPANEDIOL (UNII: N8F53B3R4R) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SILANEDIOL SALICYLATE (UNII: C054DF30K0) TROMETHAMINE (UNII: 023C2WHX2V) CERAMIDE NP (UNII: 4370DF050B) SODIUM BENZOATE (UNII: OJ245FE5EU) PROLINE (UNII: 9DLQ4CIU6V) CALCIUM CITRATE (UNII: MLM29U2X85) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) MAITAKE (UNII: A1U5YJI0Z8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LAURETH-12 (UNII: OAH19558U1) SODIUM ALGINATE (UNII: C269C4G2ZQ) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) AMINO ACIDS, RICE (UNII: 5ET1T25H82) ADANSONIA DIGITATA SEED (UNII: 2936P60TPX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PANTHENOL (UNII: WV9CM0O67Z) HYALURONIC ACID (UNII: S270N0TRQY) ACACIA (UNII: 5C5403N26O) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-330-01 1 in 1 BOX 04/19/2023 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 04/19/2023 LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 CHAMPAGNE 170-N
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-326 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ISODODECANE (UNII: A8289P68Y2) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) CHLORPHENESIN (UNII: I670DAL4SZ) NYLON-12 (UNII: 446U8J075B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERRIC OXIDE RED (UNII: 1K09F3G675) GLYCERIN (UNII: PDC6A3C0OX) NIACINAMIDE (UNII: 25X51I8RD4) MALTODEXTRIN (UNII: 7CVR7L4A2D) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PEG-10 DIMETHICONE (220 CST) (UNII: 287GF3Y3WC) POLYMETHYLSILSESQUIOXANE/TRIMETHYLSILOXYSILICATE (UNII: X2PZH4Y6HT) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYLPROPANEDIOL (UNII: N8F53B3R4R) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SILANEDIOL SALICYLATE (UNII: C054DF30K0) TROMETHAMINE (UNII: 023C2WHX2V) CERAMIDE NP (UNII: 4370DF050B) SODIUM BENZOATE (UNII: OJ245FE5EU) PROLINE (UNII: 9DLQ4CIU6V) CALCIUM CITRATE (UNII: MLM29U2X85) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) MAITAKE (UNII: A1U5YJI0Z8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LAURETH-12 (UNII: OAH19558U1) SODIUM ALGINATE (UNII: C269C4G2ZQ) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) AMINO ACIDS, RICE (UNII: 5ET1T25H82) ADANSONIA DIGITATA SEED (UNII: 2936P60TPX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PANTHENOL (UNII: WV9CM0O67Z) HYALURONIC ACID (UNII: S270N0TRQY) ACACIA (UNII: 5C5403N26O) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-326-01 1 in 1 BOX 04/19/2023 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 04/19/2023 LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 ALMENDRA 230-N
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-329 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ISODODECANE (UNII: A8289P68Y2) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) CHLORPHENESIN (UNII: I670DAL4SZ) NYLON-12 (UNII: 446U8J075B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERRIC OXIDE RED (UNII: 1K09F3G675) GLYCERIN (UNII: PDC6A3C0OX) NIACINAMIDE (UNII: 25X51I8RD4) MALTODEXTRIN (UNII: 7CVR7L4A2D) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PEG-10 DIMETHICONE (220 CST) (UNII: 287GF3Y3WC) POLYMETHYLSILSESQUIOXANE/TRIMETHYLSILOXYSILICATE (UNII: X2PZH4Y6HT) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYLPROPANEDIOL (UNII: N8F53B3R4R) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SILANEDIOL SALICYLATE (UNII: C054DF30K0) TROMETHAMINE (UNII: 023C2WHX2V) CERAMIDE NP (UNII: 4370DF050B) SODIUM BENZOATE (UNII: OJ245FE5EU) PROLINE (UNII: 9DLQ4CIU6V) CALCIUM CITRATE (UNII: MLM29U2X85) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) MAITAKE (UNII: A1U5YJI0Z8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LAURETH-12 (UNII: OAH19558U1) SODIUM ALGINATE (UNII: C269C4G2ZQ) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) AMINO ACIDS, RICE (UNII: 5ET1T25H82) ADANSONIA DIGITATA SEED (UNII: 2936P60TPX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PANTHENOL (UNII: WV9CM0O67Z) HYALURONIC ACID (UNII: S270N0TRQY) ACACIA (UNII: 5C5403N26O) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-329-01 1 in 1 BOX 04/19/2023 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 04/19/2023 LBEL CONCENTRE BASE SERUM ANTIEDAD EFECTO LIFTING FPS 15 ANTI AGING LIFTING SERUM FOUNDATION SPF 15 LATTE 180-F
octinoxate, zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-327 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 29.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ISODODECANE (UNII: A8289P68Y2) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) ALCOHOL (UNII: 3K9958V90M) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROXYETHYL UREA (UNII: MBQ7DDQ7AR) CHLORPHENESIN (UNII: I670DAL4SZ) NYLON-12 (UNII: 446U8J075B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERRIC OXIDE RED (UNII: 1K09F3G675) GLYCERIN (UNII: PDC6A3C0OX) NIACINAMIDE (UNII: 25X51I8RD4) MALTODEXTRIN (UNII: 7CVR7L4A2D) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PEG-10 DIMETHICONE (220 CST) (UNII: 287GF3Y3WC) POLYMETHYLSILSESQUIOXANE/TRIMETHYLSILOXYSILICATE (UNII: X2PZH4Y6HT) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYLPROPANEDIOL (UNII: N8F53B3R4R) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SILANEDIOL SALICYLATE (UNII: C054DF30K0) TROMETHAMINE (UNII: 023C2WHX2V) CERAMIDE NP (UNII: 4370DF050B) SODIUM BENZOATE (UNII: OJ245FE5EU) PROLINE (UNII: 9DLQ4CIU6V) CALCIUM CITRATE (UNII: MLM29U2X85) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) MAITAKE (UNII: A1U5YJI0Z8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LAURETH-12 (UNII: OAH19558U1) SODIUM ALGINATE (UNII: C269C4G2ZQ) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) AMINO ACIDS, RICE (UNII: 5ET1T25H82) ADANSONIA DIGITATA SEED (UNII: 2936P60TPX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PANTHENOL (UNII: WV9CM0O67Z) HYALURONIC ACID (UNII: S270N0TRQY) ACACIA (UNII: 5C5403N26O) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-327-01 1 in 1 BOX 04/19/2023 1 26 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 04/19/2023 Labeler - BEL STAR SA (880160197)