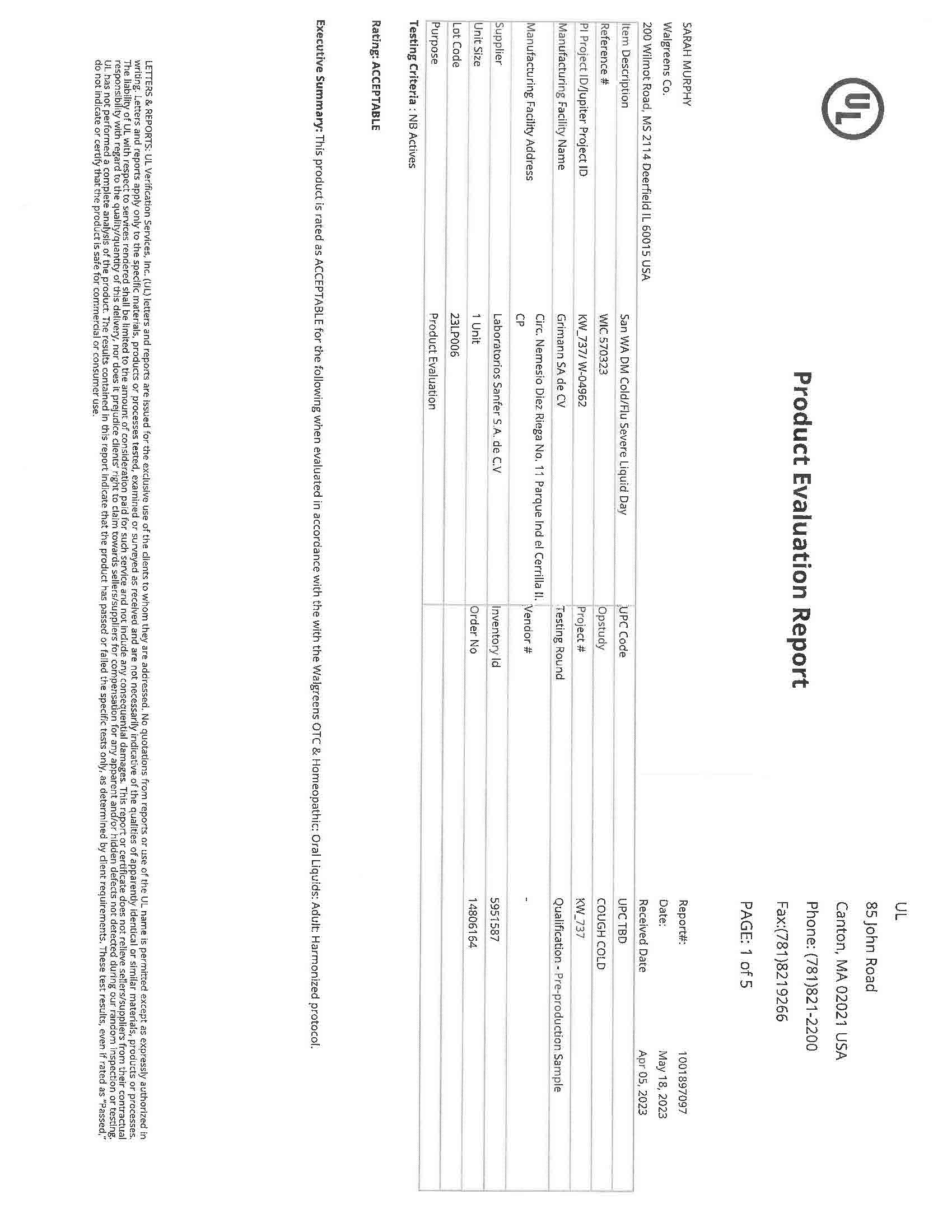
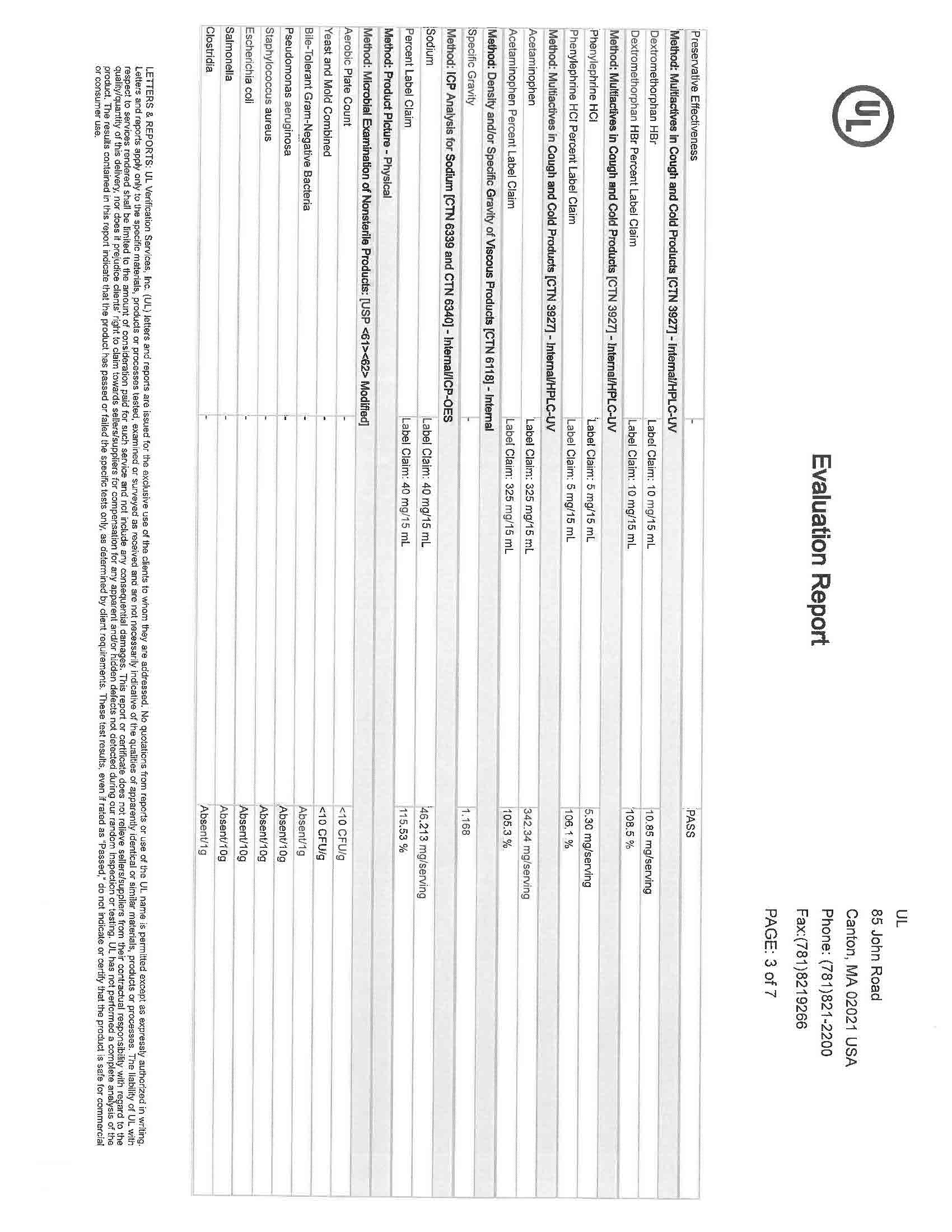
Label: NIGHTTIME COLD AND FLU- acetaminophen, dextromethorphan, doxylamine succinate liquid
NIGHTTIME COLD AND FLU LIQUID CHERRY- acetaminophen, dextromethorphan, doxylamine succinate liquid
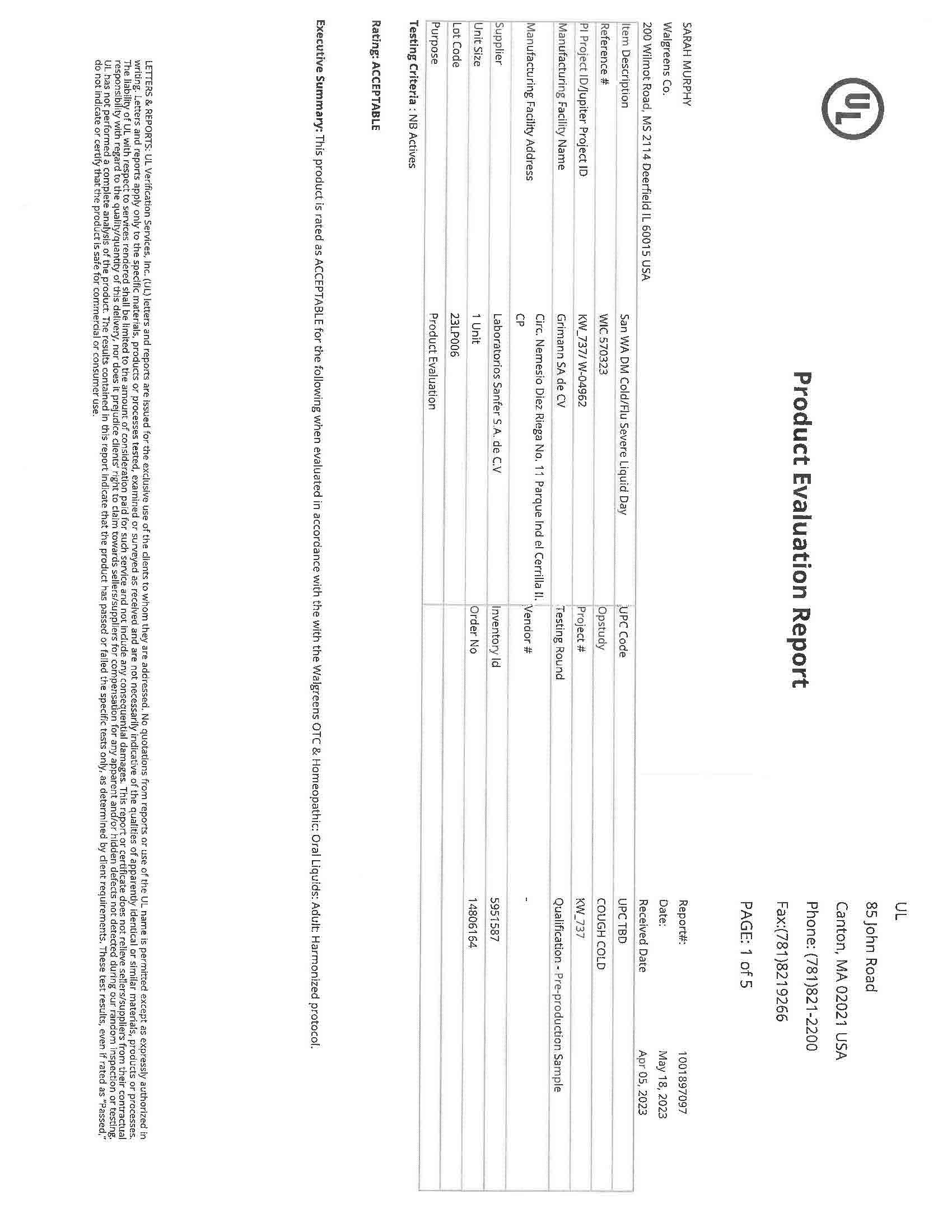
- NDC Code(s): 83393-555-01, 83393-777-01
- Packager: Laboratorios Sanfer, S.A. de C.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
-
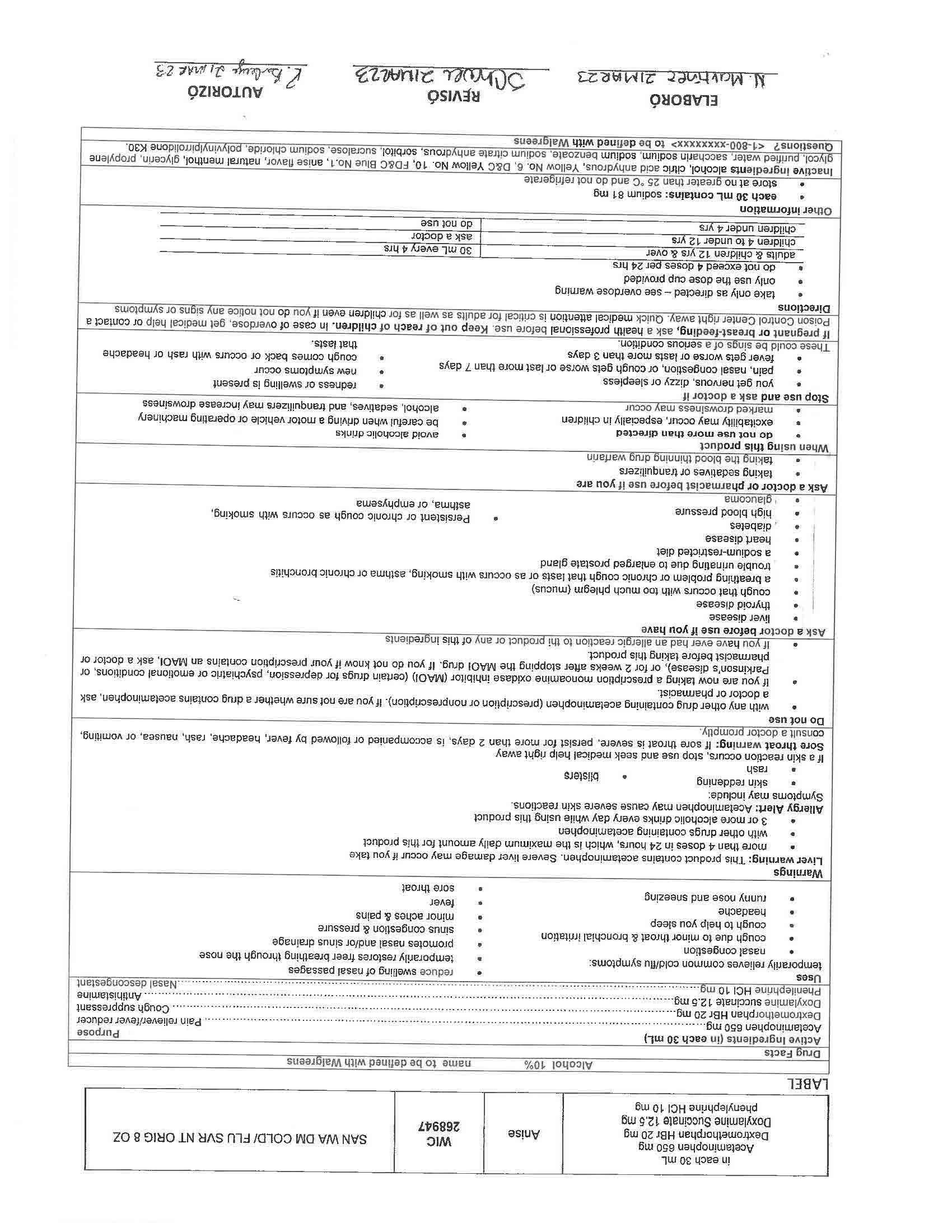
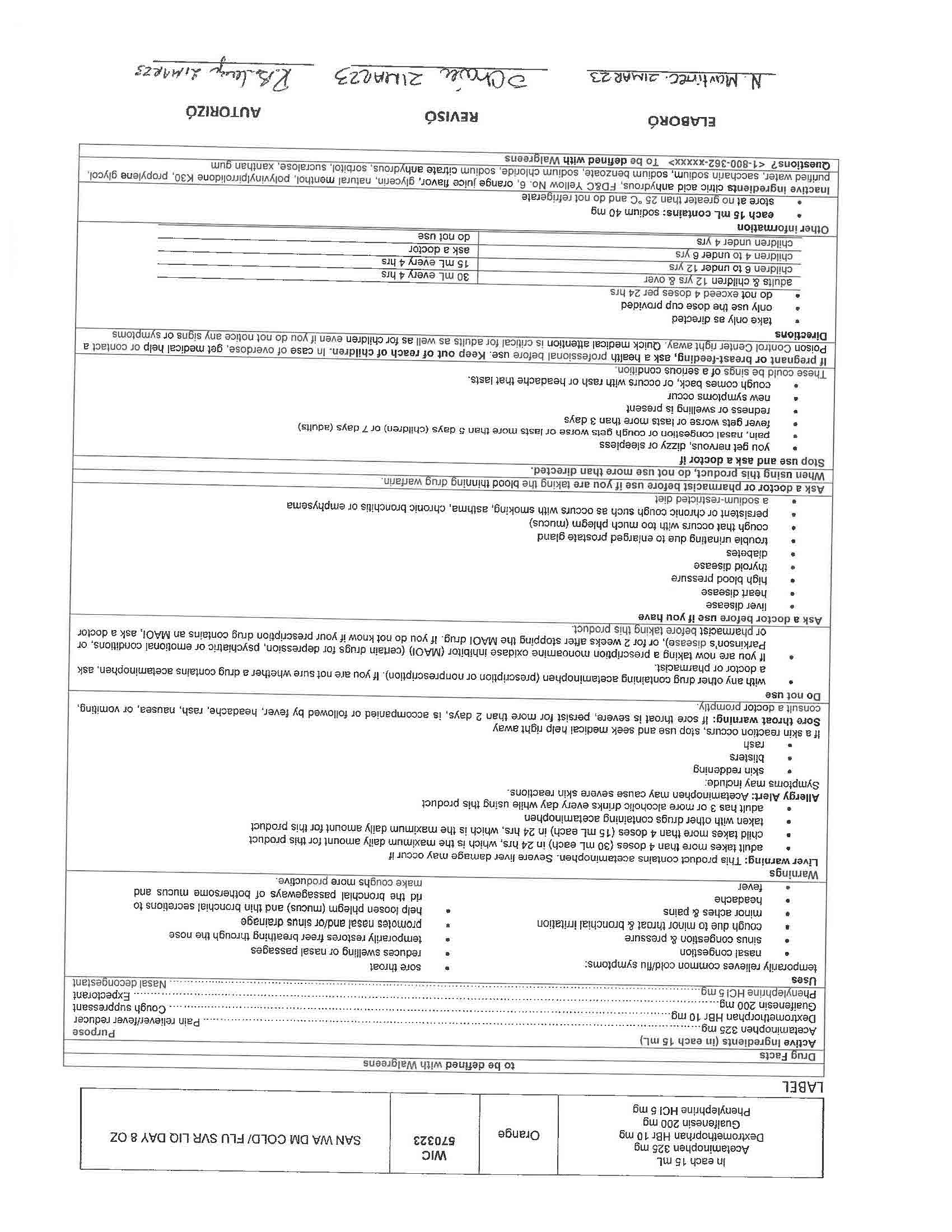
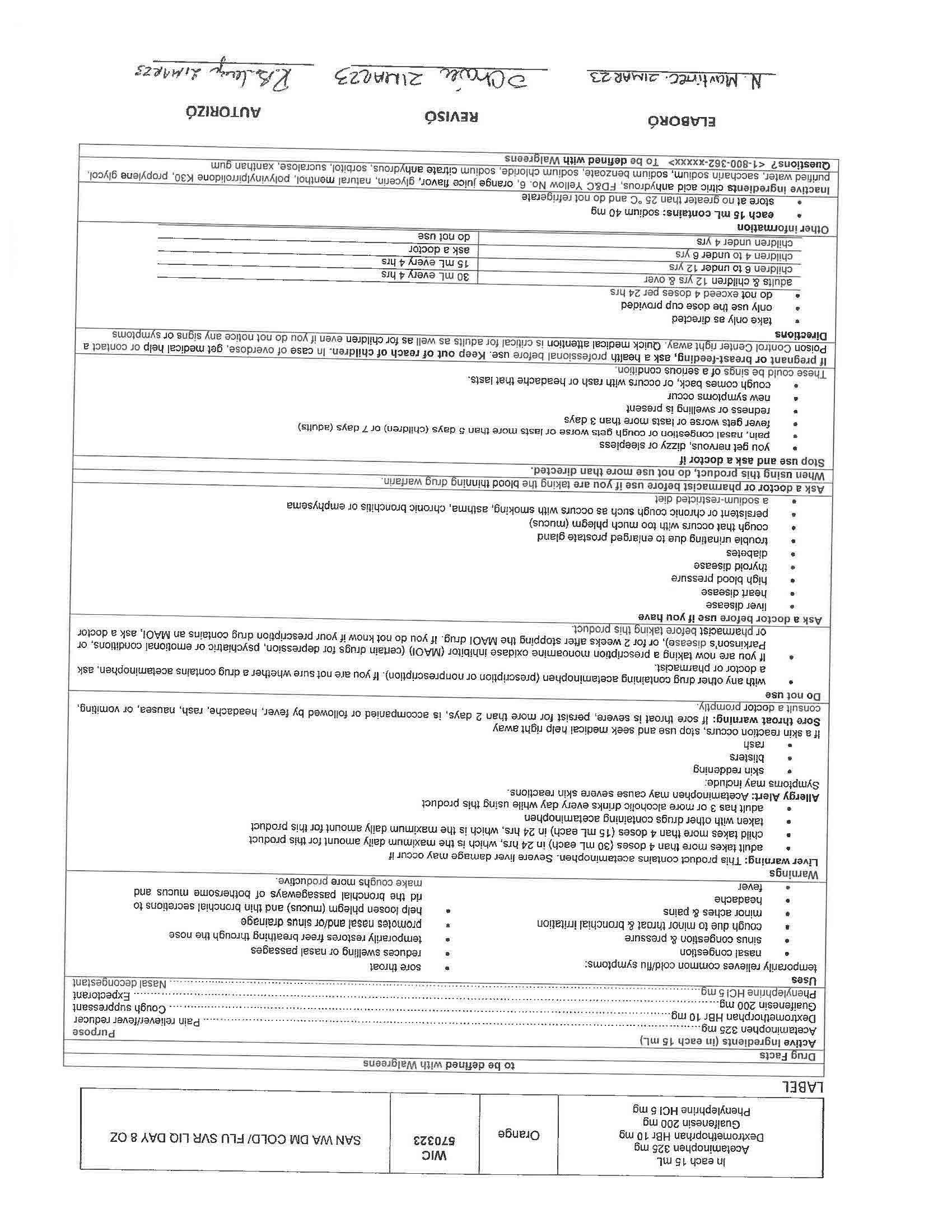
WARNINGS
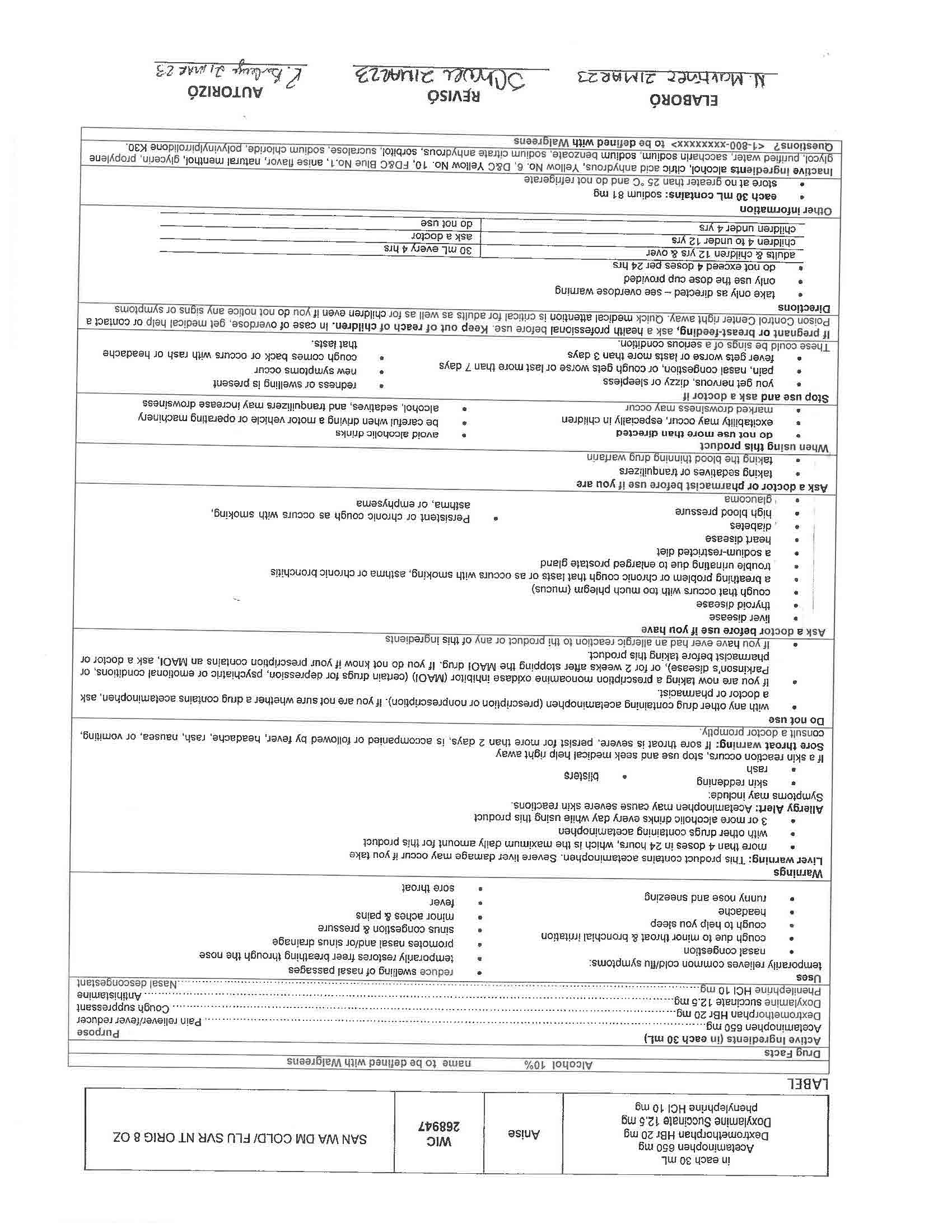
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
more than 4 doses in 24 hrs, which is the maximum daily amount for this product
with other drugs containing acetaminophen
3 or more alcoholic drinks every day while using this productAllergy Alert: Acetaminophen may cause severe skin reactions.
Symptoms may include:
skin reddening
blisters
rashIf skin reaction occurs, stop use and seek medical help right away
Sore throat warning: If sore throat is severe, persist for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
-
KEEP OUT OF REACH OF CHILDREN
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms
- PURPOSE
-
ACTIVE INGREDIENT
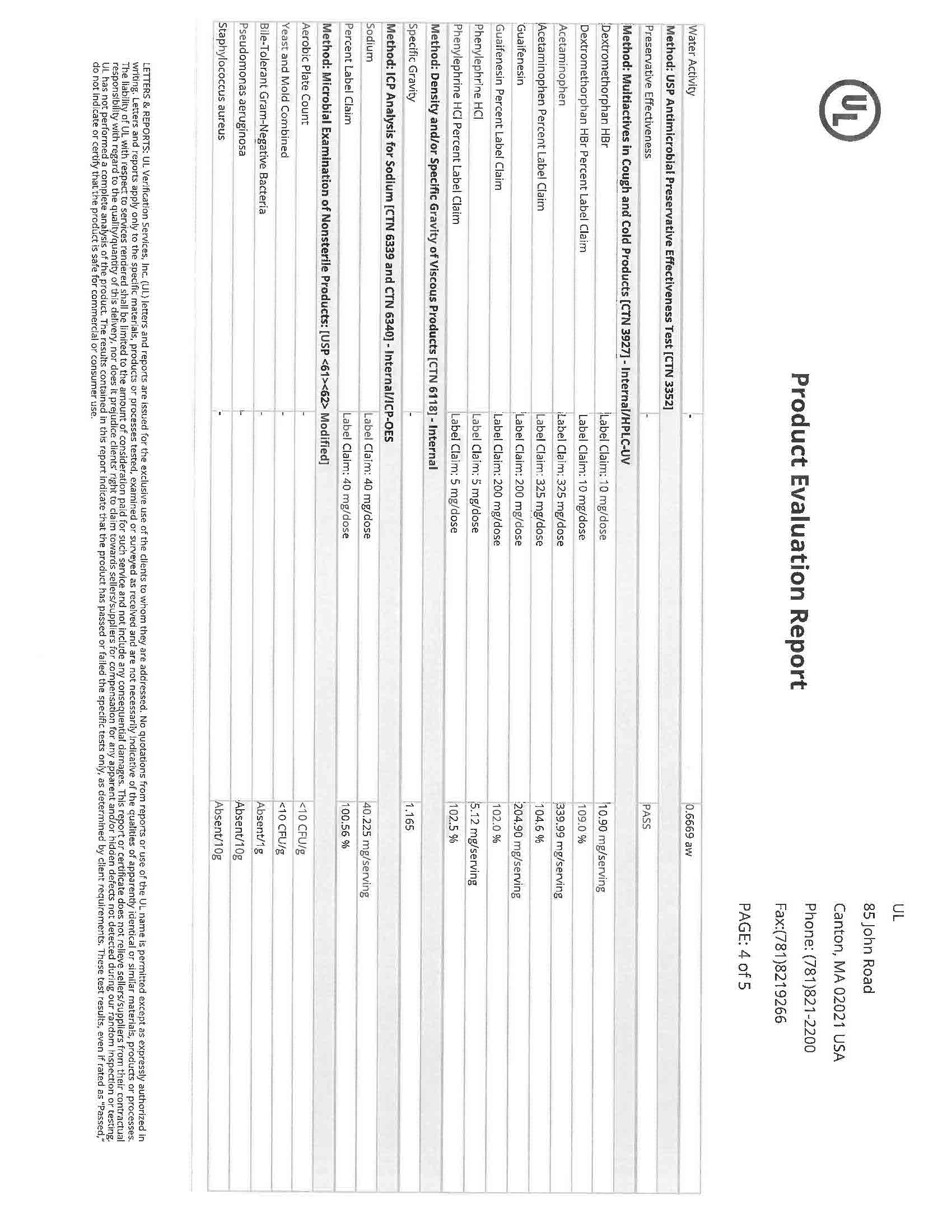
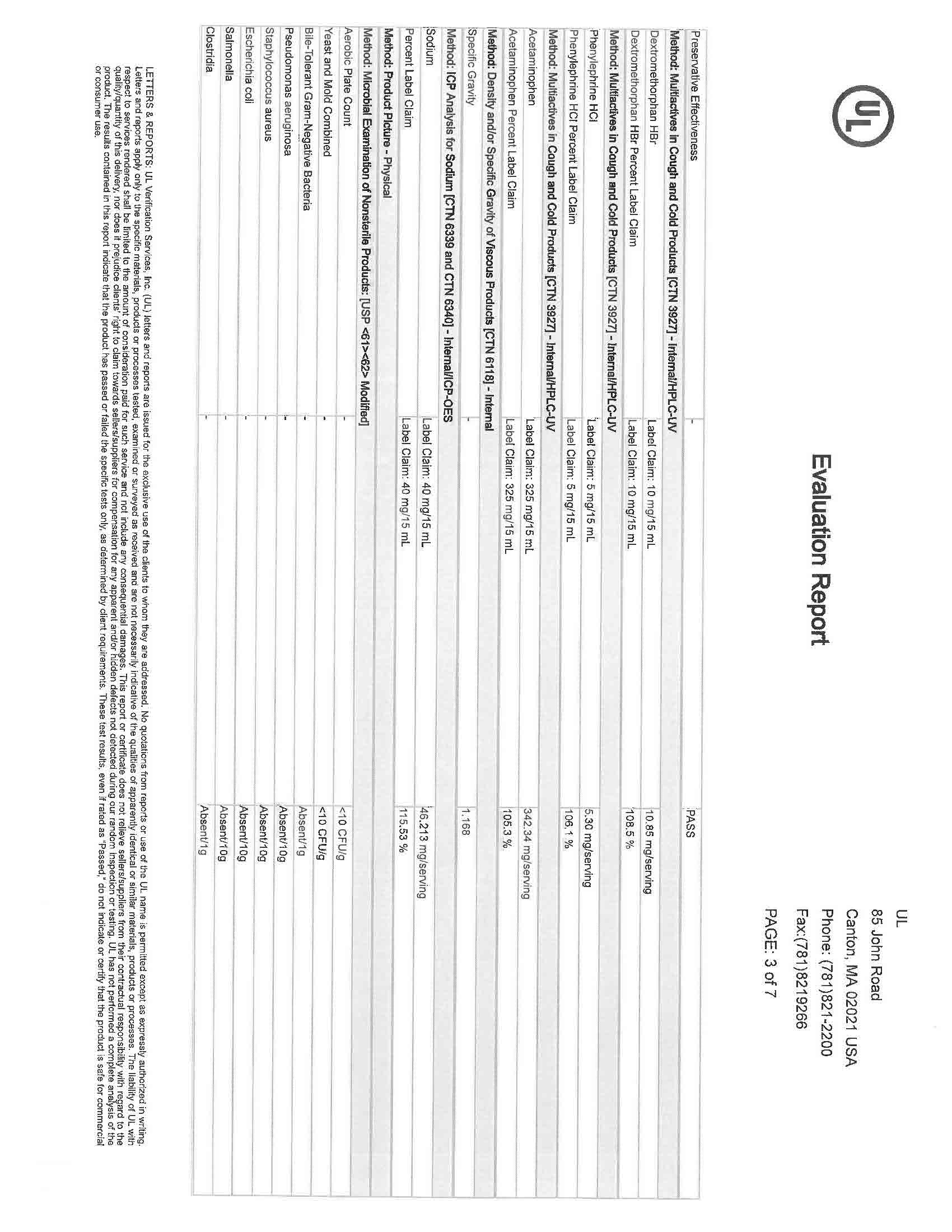
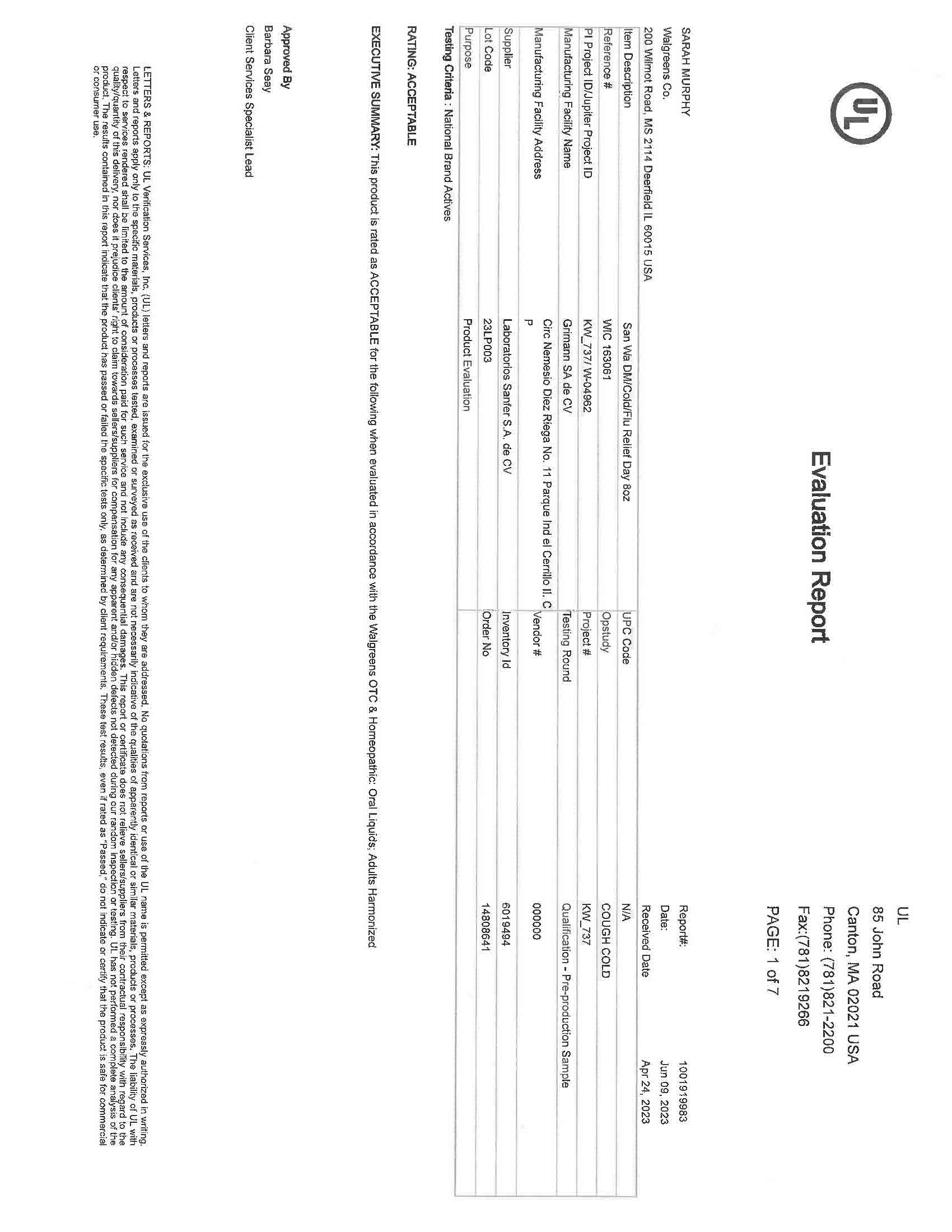
Active Ingredients (in each 30 mL) Purpose
Acetaminophen 650 mg……………………………………………………………………………………………………………….……….. Pain reliever/fever reducer
Dextromethorphan HBr 30 mg…………………………………………………………………………………………………….….………….……. Cough suppressant
Doxylamine succinate 12.5 mg………………………………………………………………………………………………………………………….……. Antihistamine
- PRINCIPAL DISPLAY PANEL
-
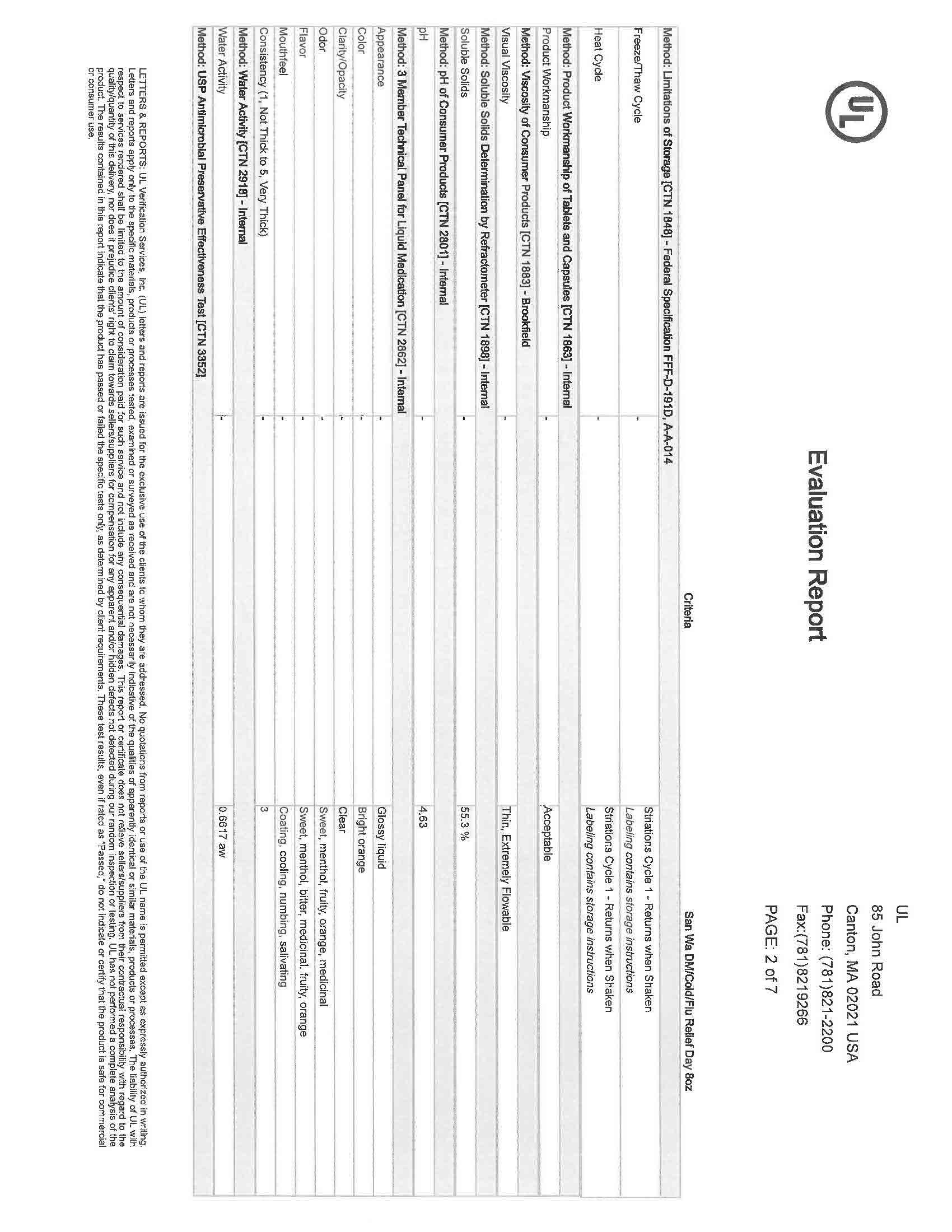
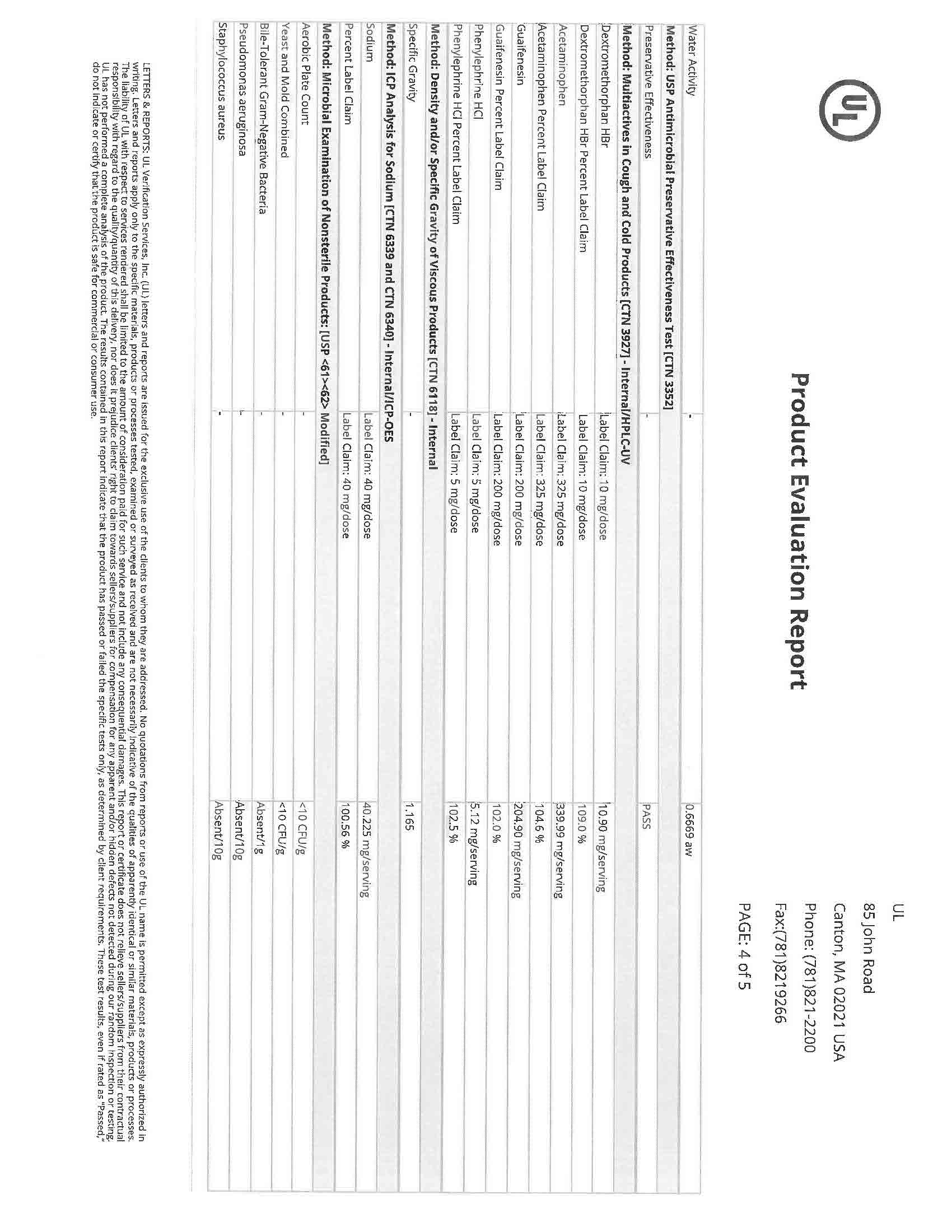
INGREDIENTS AND APPEARANCE
NIGHTTIME COLD AND FLU
acetaminophen, dextromethorphan, doxylamine succinate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83393-777 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 30 mL DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 12.5 mg in 30 mL ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 1 mg in 30 mL SACCHARIN SODIUM (UNII: SB8ZUX40TY) 1 mg in 30 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 1 mg in 30 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) 1 mg in 30 mL SUCRALOSE (UNII: 96K6UQ3ZD4) 1 mg in 30 mL PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 1 mg in 30 mL ALCOHOL (UNII: 3K9958V90M) 1 mg in 30 mL D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) 1 mg in 30 mL GLYCERIN (UNII: PDC6A3C0OX) 1 mg in 30 mL SORBITOL (UNII: 506T60A25R) 1 mg in 30 mL CITRIC ACID ACETATE (UNII: DSO12WL7AU) 1 mg in 30 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 1 mg in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83393-777-01 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 04/11/2023 NIGHTTIME COLD AND FLU LIQUID CHERRY
acetaminophen, dextromethorphan, doxylamine succinate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83393-555 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 30 mL DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 12.5 mg in 30 mL ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 1 mg in 30 mL SACCHARIN SODIUM (UNII: SB8ZUX40TY) 1 mg in 30 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 1 mg in 30 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) 1 mg in 30 mL SUCRALOSE (UNII: 96K6UQ3ZD4) 1 mg in 30 mL ALCOHOL (UNII: 3K9958V90M) 1 mg in 30 mL D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) 1 mg in 30 mL GLYCERIN (UNII: PDC6A3C0OX) 1 mg in 30 mL SORBITOL (UNII: 506T60A25R) 1 mg in 30 mL CITRIC ACID ACETATE (UNII: DSO12WL7AU) 1 mg in 30 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 1 mg in 30 mL PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 1 mg in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83393-555-01 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/11/2023 Labeler - Laboratorios Sanfer, S.A. de C.V. (810007732) Establishment Name Address ID/FEI Business Operations Laboratorios Sanfer, S.A. de C.V. 810007732 manufacture(83393-777, 83393-555)