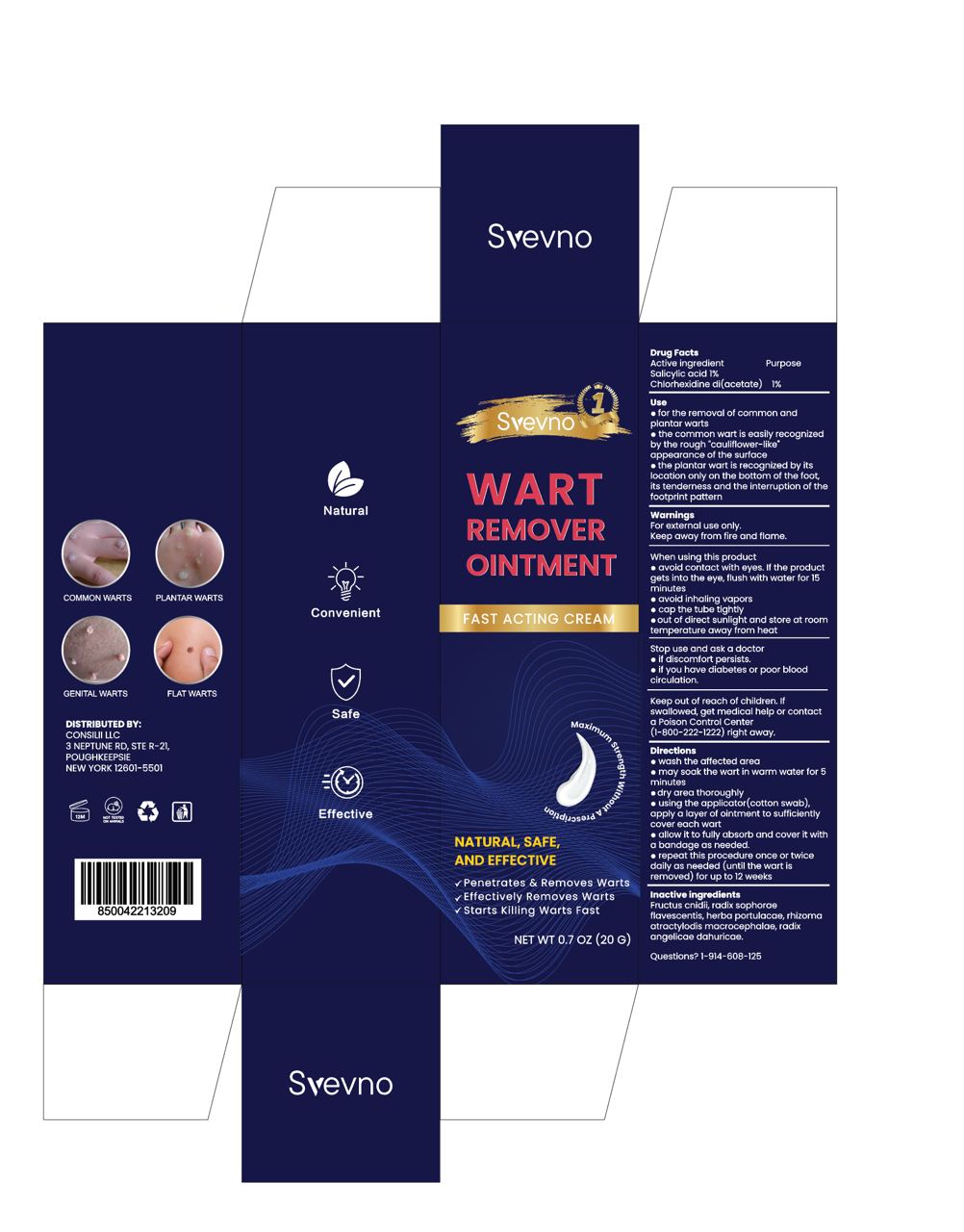
Label: WART REMOVER OINTMENT.- you yuping antibacterial ointment ointment
- NDC Code(s): 83299-002-01
- Packager: Consilii LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING SECTION
- STOP USE section
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
Directions
wash the affected area
may soak the wart in warm water for 5minutes
dry area thoroughly.
using the applicator(cotton swab)apply a layer of ointment to sufficiently cover each wart
aliow it to fully absorb and cover it witha bandage as needed
repeat this procedure once or twicedaily as needed (until the wart isremoved) for up to 12 weeks - Other information
- Inactive ingredients
- QUESTIONS
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WART REMOVER OINTMENT.
you yuping antibacterial ointment ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83299-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1 g in 100 g CHLORHEXIDINE (UNII: R4KO0DY52L) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE 1 g in 100 g Inactive Ingredients Ingredient Name Strength ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) CITRUS RETICULATA WHOLE (UNII: O0OX7CMF92) FORSYTHIA SUSPENSA ROOT (UNII: YM9E56VP46) PLATYCODON GRANDIFLORUS WHOLE (UNII: WC73QE9274) ASARUM SIEBOLDII (UNII: T1134STW1K) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) PORTULACA OLERACEA WHOLE (UNII: D5J3623SV2) ATRACTYLODES MACROCEPHALA ROOT (UNII: 08T3N29QJB) ARCTIUM LAPPA WHOLE (UNII: 73070DU1LA) BAMBUSA VULGARIS LEAF (UNII: EMY54R518C) BORNEOL (UNII: M89NIB437X) CNIDIUM MONNIERI FRUIT (UNII: V1IA3S3CUS) HONEY (UNII: Y9H1V576FH) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83299-002-01 20 g in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M028 04/05/2023 Labeler - Consilii LLC (118891890) Establishment Name Address ID/FEI Business Operations Consilii LLC 118891890 label(83299-002) , manufacture(83299-002)