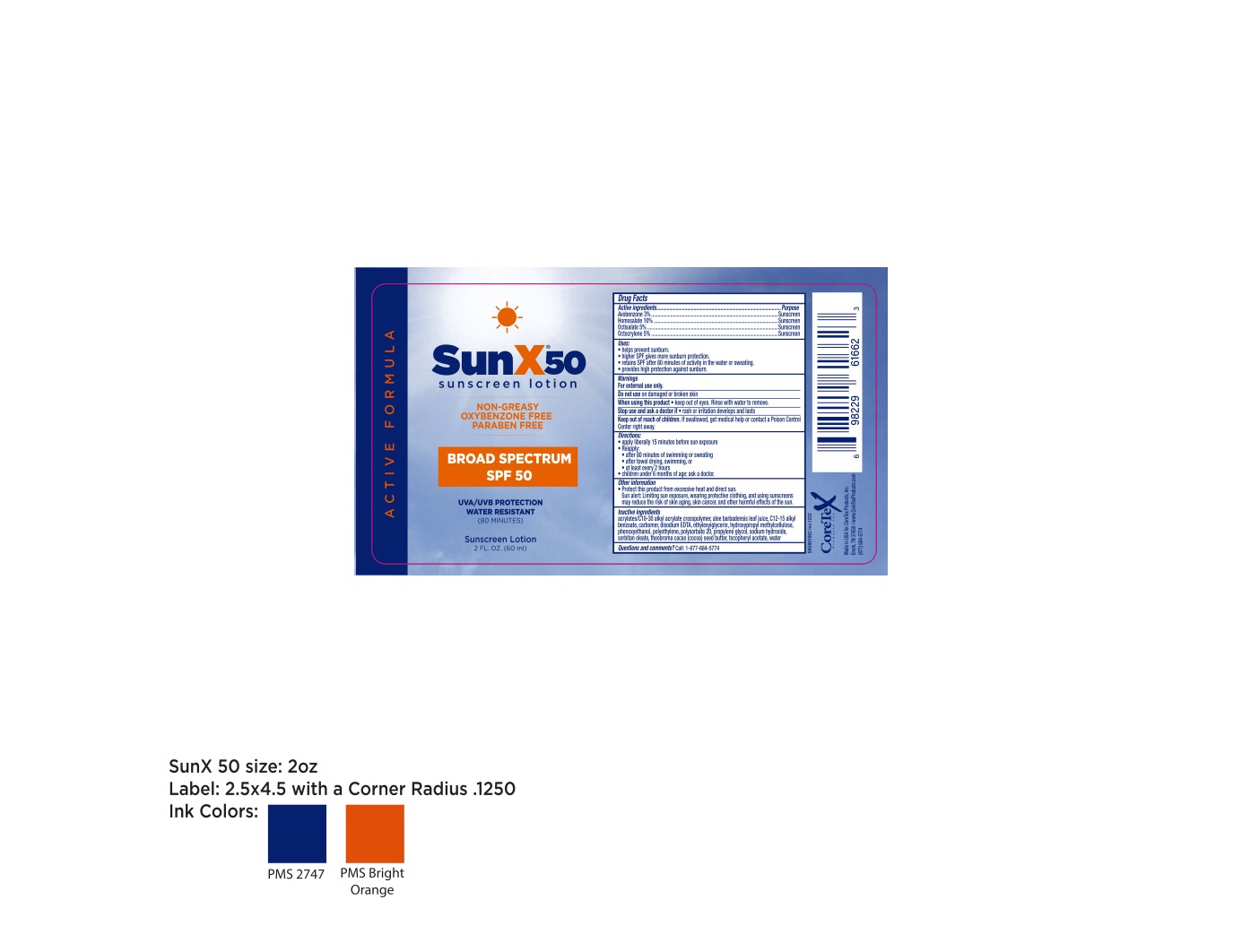
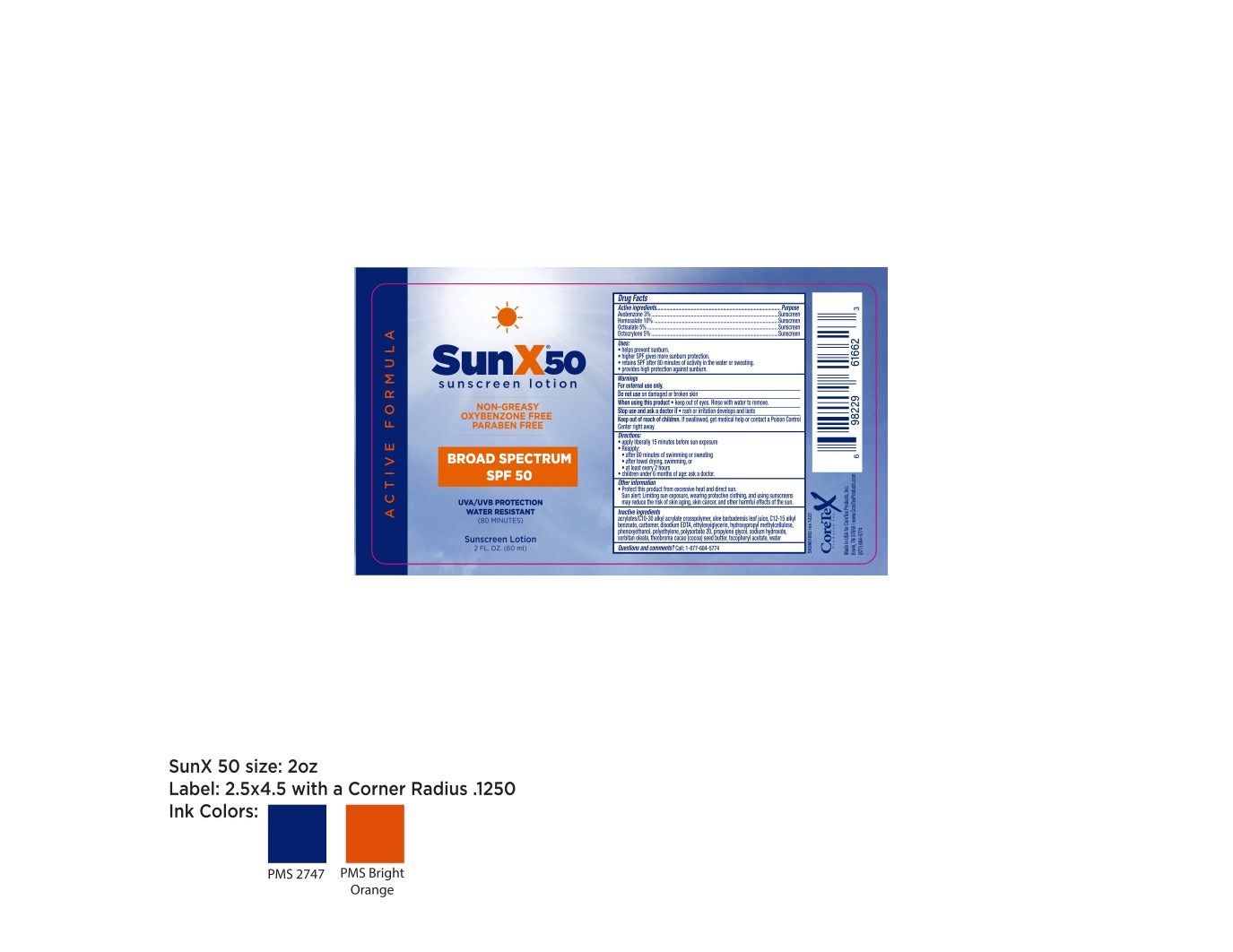
Label: CORETEX SUN X 50- avobenzone, homosalate, octisalate, octocrylene lotion
-
NDC Code(s):
65753-603-01,
65753-603-02,
65753-603-03,
65753-603-04, view more65753-603-05, 65753-603-07, 65753-603-09, 65753-603-10, 65753-603-22, 65753-603-23, 65753-603-24, 65753-603-25, 65753-603-26, 65753-603-32, 65753-603-33, 65753-603-34, 65753-603-39
- Packager: CoreTex Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses:
- Warnings
- Directions
- Other information
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, C12-15 alkyl benzoate, carbomer, disodium EDTA, ethylexylglycerin, hydroxypropyl methylcellulose phenoxyethanol, polyethylene, polysorbate 20, propylene glycol, sodium hydroxide, sorbitan oleate, theobroma cacao (cocoa) seed butter, tocopheryl acetate, water
- Questions?
- -603-34
- -603-01
- -603-02
- -603-03
- -603-04
- -603-05
- -603-07
- -603-09
- -603-10
- -603-22-23-26-39
- -603-24
- -603-25
- -603-32
- -603-33
- -603-35
-
INGREDIENTS AND APPEARANCE
CORETEX SUN X 50
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65753-603 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength ACRYLATES CROSSPOLYMER-6 (UNII: 4GXD0Q3OS3) METHYLCELLULOSE, UNSPECIFIED (UNII: Z944H5SN0H) SODIUM HYDROXIDE (UNII: 55X04QC32I) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) MEDIUM DENSITY POLYETHYLENE (UNII: 3W404QE89S) THEOBROMA CACAO WHOLE (UNII: EB048G1S9J) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PEG-6 SORBITAN OLEATE (UNII: 58O7V09UCI) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) POLYSORBATE 20 (UNII: 7T1F30V5YH) ALOE VERA LEAF (UNII: ZY81Z83H0X) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65753-603-01 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 2 NDC:65753-603-32 44 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 3 NDC:65753-603-02 60 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 4 NDC:65753-603-33 60 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 5 NDC:65753-603-03 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 6 NDC:65753-603-34 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 7 NDC:65753-603-04 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 8 NDC:65753-603-05 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 9 NDC:65753-603-07 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 10 NDC:65753-603-09 946 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 11 NDC:65753-603-10 3788 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2023 12 NDC:65753-603-39 7 mL in 1 POUCH; Type 0: Not a Combination Product 01/03/2023 13 NDC:65753-603-22 25 in 1 CONTAINER 01/03/2023 13 NDC:65753-603-39 7 mL in 1 POUCH; Type 0: Not a Combination Product 14 NDC:65753-603-23 25 in 1 CONTAINER 01/03/2023 14 NDC:65753-603-39 7 mL in 1 POUCH; Type 0: Not a Combination Product 15 NDC:65753-603-24 50 in 1 CONTAINER 01/03/2023 15 NDC:65753-603-39 7 mL in 1 POUCH; Type 0: Not a Combination Product 16 NDC:65753-603-25 100 in 1 CONTAINER 01/03/2023 16 NDC:65753-603-39 7 mL in 1 POUCH; Type 0: Not a Combination Product 17 NDC:65753-603-26 300 in 1 CARTON 01/03/2023 17 NDC:65753-603-39 7 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/03/2023 Labeler - CoreTex Products Inc (061944620) Establishment Name Address ID/FEI Business Operations CoreTex Products Inc 061944620 pack(65753-603) Establishment Name Address ID/FEI Business Operations Prime Enterprises 101946028 manufacture(65753-603)