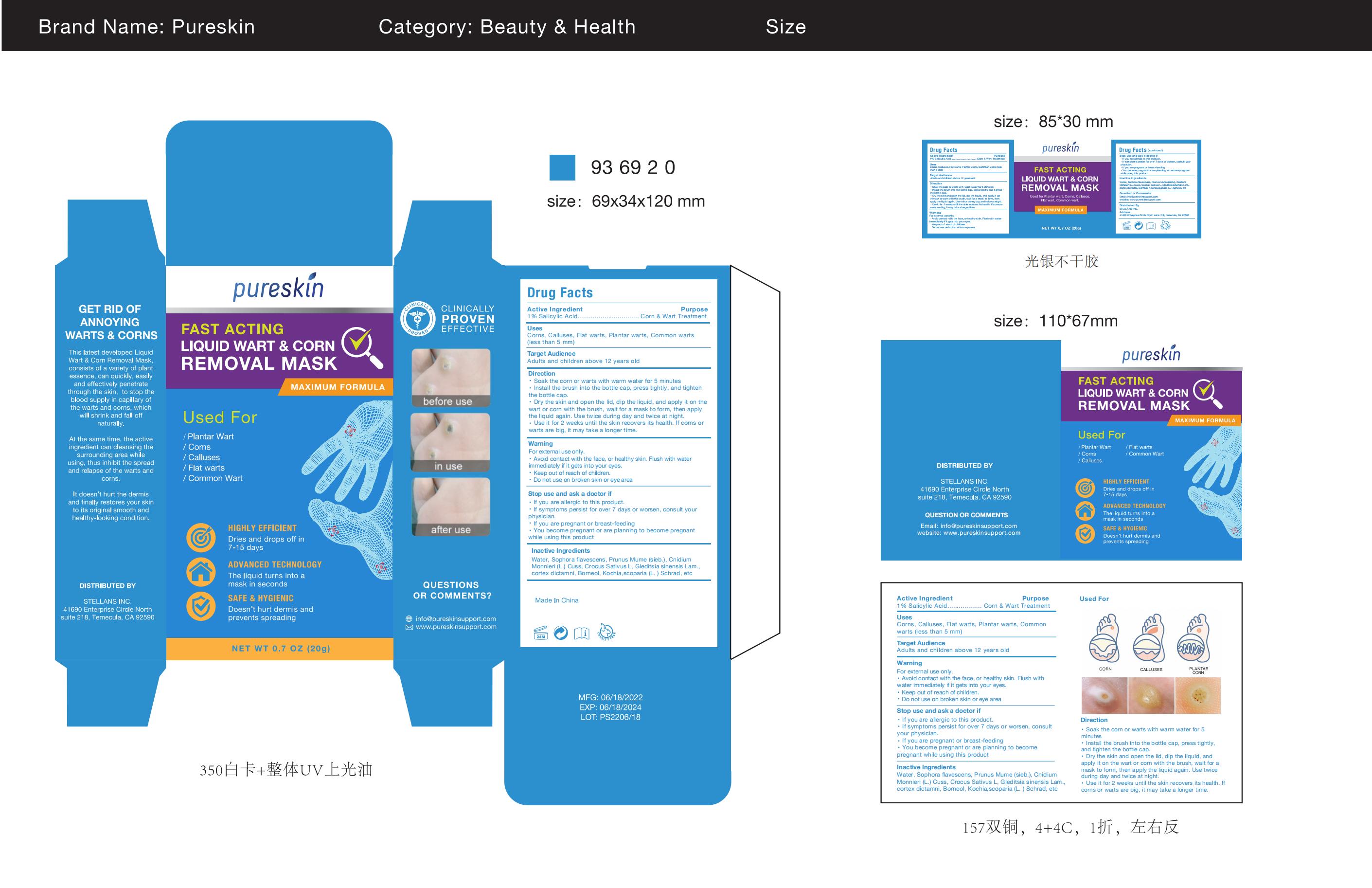
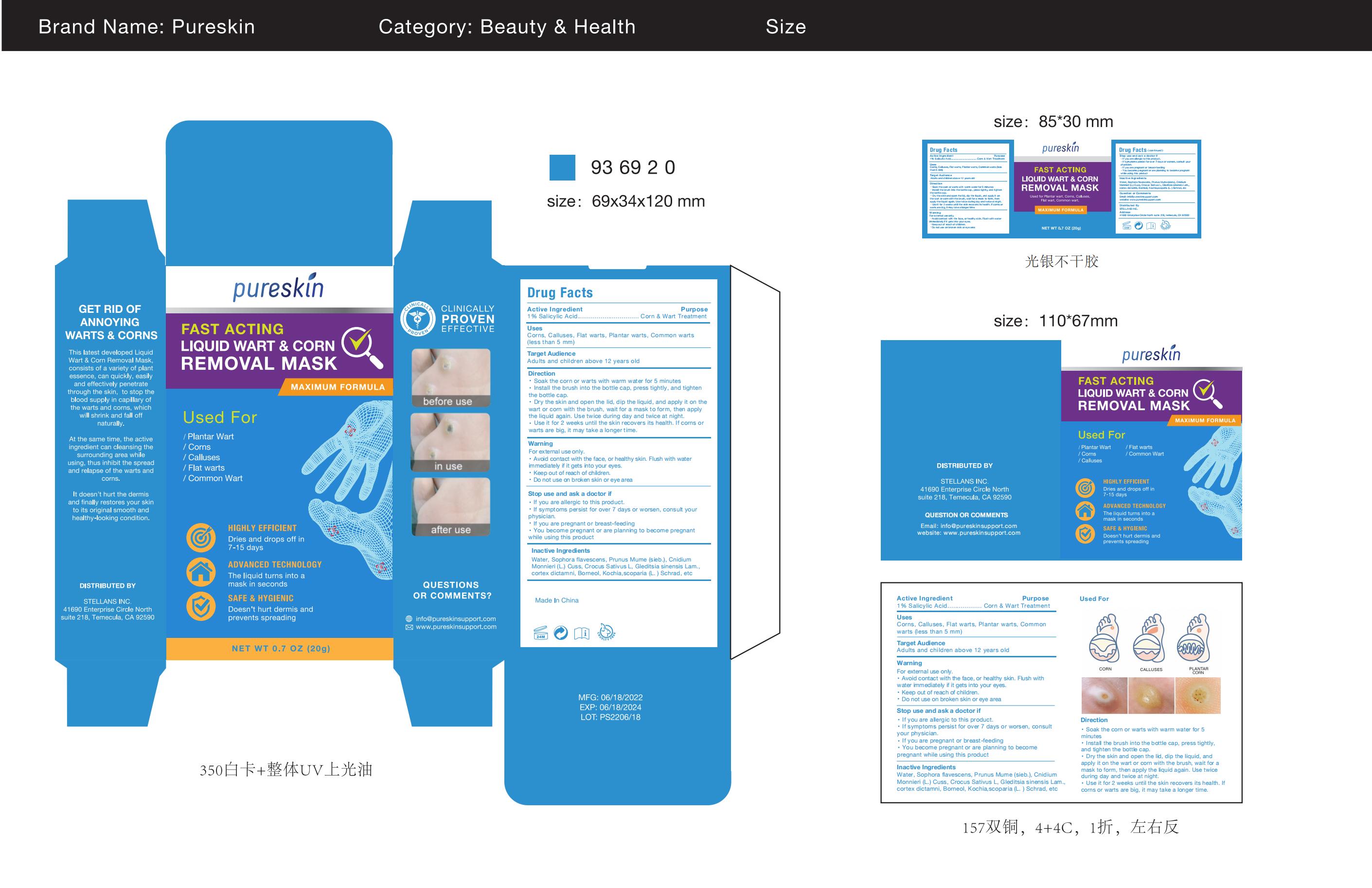
Label: PURESKIN LIQUID WART CORN REMOVAL MASK- liquid wart corn removal mask paste
- NDC Code(s): 83176-005-01
- Packager: Dr.luke Healthcare LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING SECTION
- STOP USE section
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Soak the corn or warts with warm water for 5 minutesInstall the brush into the bottle cap, press tightly, and tightenthe bottle cap.
Dry the skin and open the lid, dip the liquid, and apply it on thewart or corn with the brush, wait for a mask to form, then applythe liquid again. Use twice during day and twice at night.Use it for 2 weeks until the skin recovers its health. lf corns orwarts are big, it may take a longer time. - Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PURESKIN LIQUID WART CORN REMOVAL MASK
liquid wart corn removal mask pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83176-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) CROCUS SATIVUS WHOLE (UNII: Z5C927G4XF) BASSIA SCOPARIA WHOLE (UNII: 240G38P85Z) BORNEOL (UNII: M89NIB437X) CNIDIUM MONNIERI WHOLE (UNII: GYR30735RE) PRUNUS MUME WHOLE (UNII: 3RSQ38B1JI) DICTAMNUS DASYCARPUS ROOT (UNII: 6153LEN214) GLEDITSIA SINENSIS WHOLE (UNII: FS3UB95UTG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83176-005-01 20 g in 1 BOX; Type 0: Not a Combination Product 03/28/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M028 03/28/2023 Labeler - Dr.luke Healthcare LLC (118868014) Establishment Name Address ID/FEI Business Operations Dr.luke Healthcare LLC 118868014 manufacture(83176-005) , label(83176-005)