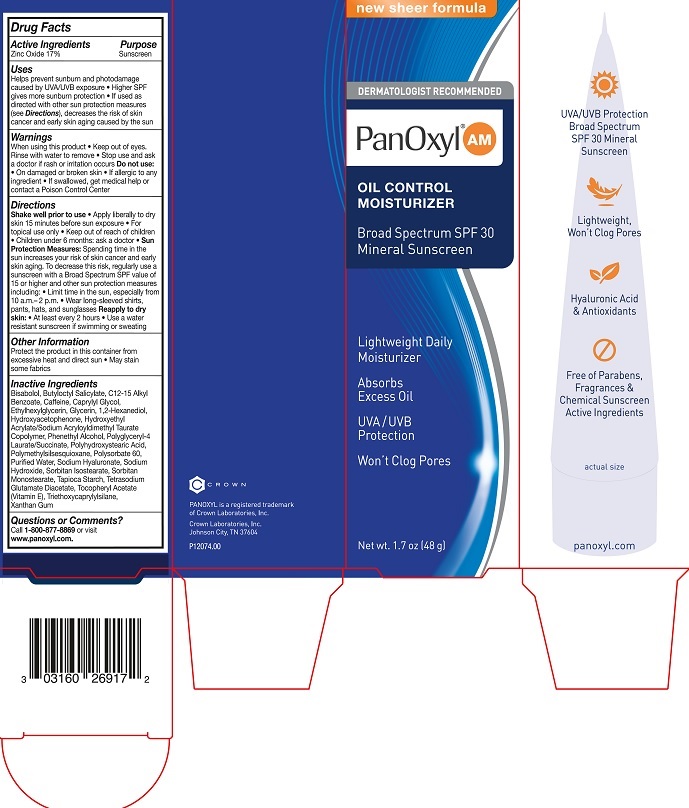
Label: PANOXYL OIL AM CONTROL MOISTURIZER- zinc oxide lotion
- NDC Code(s): 0316-0269-17
- Packager: Crown Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
-
Uses
- Helps prevent sunburn and photodamage caused by UVA/UVB exposure
- Higher SPF gives more sunburn protection
- If used as directed with other sun protection measures (see Directions), decreases the risks of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
• Shake well prior to use
• Apply liberally to dry skin 15 minutes before sun exposure
• For topical use only
• Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • Limit time in the sun, especially from 10 a.m. - 2 p.m.
• Wear long-sleeved shirts, pants, hats, and sunglasses.
• Reapply to dry skin: • At least every 2 hours • Use a water resistant suscreen if swimming for sweating
- Other Information
-
Inactive Ingredients
Bisabolol, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Caffeine, Caprylyl Glycol, Ethylhexylglycerin, Glycerin, 1,2-Hexanediol, Hydroxyacetophenone, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Phenethyl Alcohol, Polyglyceryl-4 Laurate/Succinate, Polyhydroxystearic Acid, Polymethylsilsesquioxane, Polysorbate 60, Purified Water, Sodium Hyaluronate, Sodium Hydroxide, Sorbitan Isostearate, Sorbitan Monostearate, Tapioca Starch, Tetrasodium Glutamate Diacetate, Tocopheryl Acetate (Vitamin E),Triethoxycaprylylsilane, Xanthan Gum
- Questions or Comments?
- PanOxyl Oil Control Moisturizer 1.7oz Tube
- PanOxyl Oil Control Moisturizer 1.7oz Carton
-
INGREDIENTS AND APPEARANCE
PANOXYL OIL AM CONTROL MOISTURIZER
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0316-0269 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 170 mg in 1 g Inactive Ingredients Ingredient Name Strength SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) STARCH, TAPIOCA (UNII: 24SC3U704I) LEVOMENOL (UNII: 24WE03BX2T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CAFFEINE (UNII: 3G6A5W338E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYGLYCERYL-4 LAURATE/SUCCINATE (UNII: KAB6DS9SBS) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) POLYSORBATE 60 (UNII: CAL22UVI4M) WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0316-0269-17 1 in 1 CARTON 01/13/2023 1 48 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/13/2023 Labeler - Crown Laboratories (079035945) Establishment Name Address ID/FEI Business Operations Crown Laboratories 079035945 manufacture(0316-0269)