Label: LORATADINE- loratadine oral solution
- NDC Code(s): 21130-042-24
- Packager: Better Living Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- use only with enclosed dosing cup
adults and children 6 years and over
2 teaspoonfuls (tsp) daily; do not take more than 2 teaspoonfuls (tsp) in 24 hours
children 2 to under 6 years of age
1 teaspoonful (tsp) daily; do not take more than 1 teaspoonful (tsp) in 24 hours
children under 2 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg/5 mL (120 mL Bottle)
NDC 21130-042-24
Signature
care®
Quality Guaranteed
Non-Drowsy*
AGES 2 YEARS & OLDERLORATADINE
ORAL SOLUTION USP
5 mg/5 mL
Antihistamine
24 Hour Relief of:
• Sneezing
• Runny Nose
• Itchy, Watery Eyes
• Itchy Throat or Nose
Contains sodium metabisulfite,
a sulfite that may cause
allergic-type reactions.
*When taken as directed.
See Drug Facts Panel.
Do not use if carton is opened, or if cap safety seal
is broken or missing.
Indoor & Outdoor Allergies
• Dye-Free • Sugar-Free • Alcohol-Free
GRAPE FLAVOR 4 FL OZ (120 mL)

-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg/5 mL Carton (120 mL)
Compare to
Children's Claritin®
active ingredient
NDC 21130-042-24
Signature
care®
Quality Guaranteed
Non-Drowsy*
AGES 2 YEARS & OLDERLORATADINE
ORAL SOLUTION
USP 5 mg/5 mL
Antihistamine
GRAPE FLAVOR
24 Hour Relief of:
• Sneezing
• Runny Nose
• Itchy, Watery Eyes
• Itchy Throat or Nose
Indoor & Outdoor Allergies
• DYE-FREE
Contains sodium metabisulfite,
a sulfite that may cause
allergic-type reactions.
Dosing Cup Included
*When taken as directed.
See Drug Facts Panel.
4 FL OZ (120 mL)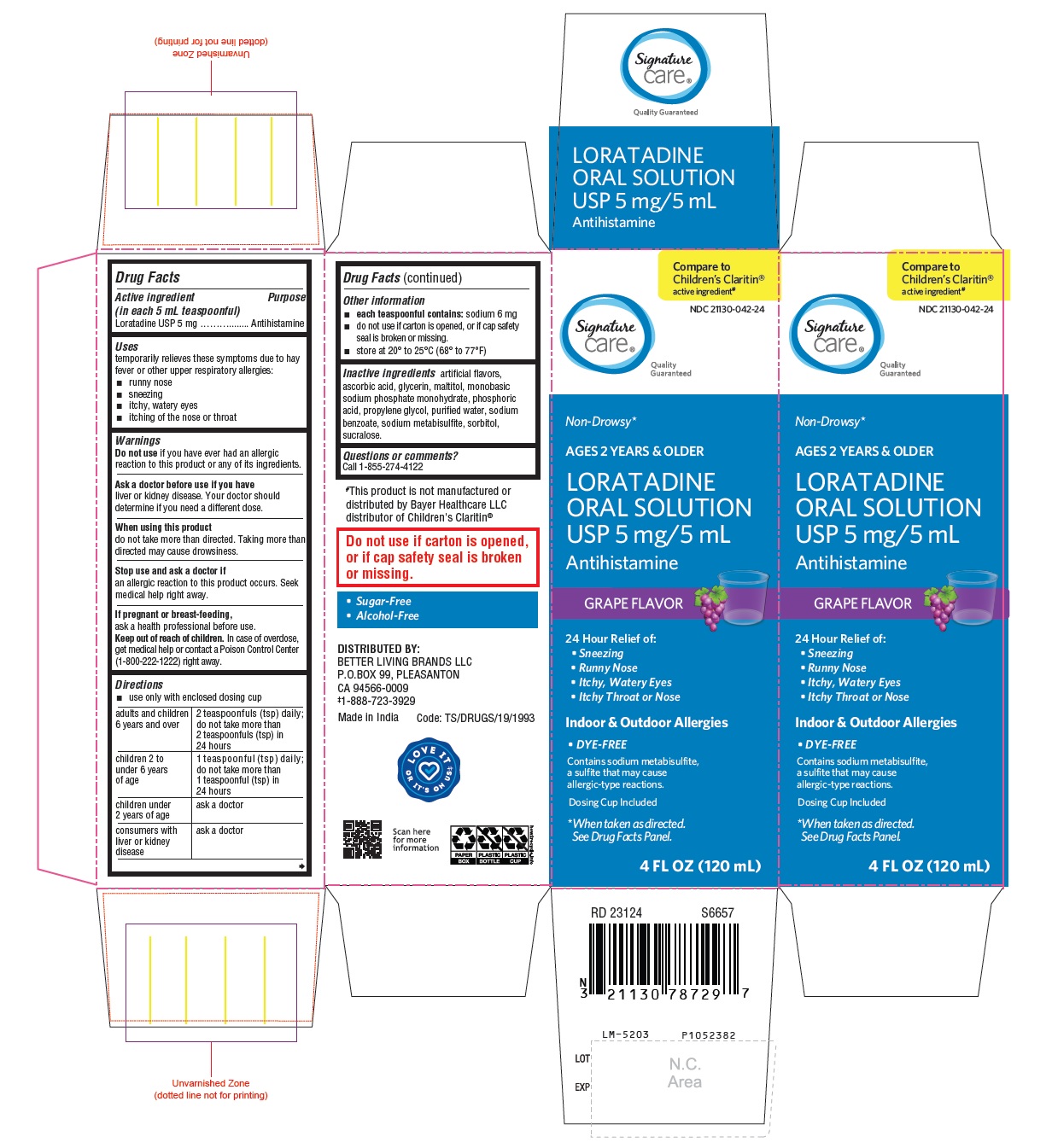
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine oral solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-042 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength GRAPE (UNII: 6X543N684K) ASCORBIC ACID (UNII: PQ6CK8PD0R) GLYCERIN (UNII: PDC6A3C0OX) MALTITOL (UNII: D65DG142WK) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) PHOSPHORIC ACID (UNII: E4GA8884NN) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color YELLOW (colorless to light yellow) Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-042-24 1 in 1 CARTON 09/25/2023 1 120 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208931 09/25/2023 Labeler - Better Living Brands LLC (009137209) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917642 ANALYSIS(21130-042) , MANUFACTURE(21130-042)


