Label: CLINICLEAN- chlorhexidine gluconate 4% solution
- NDC Code(s): 69464-901-04, 69464-901-15
- Packager: HR Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
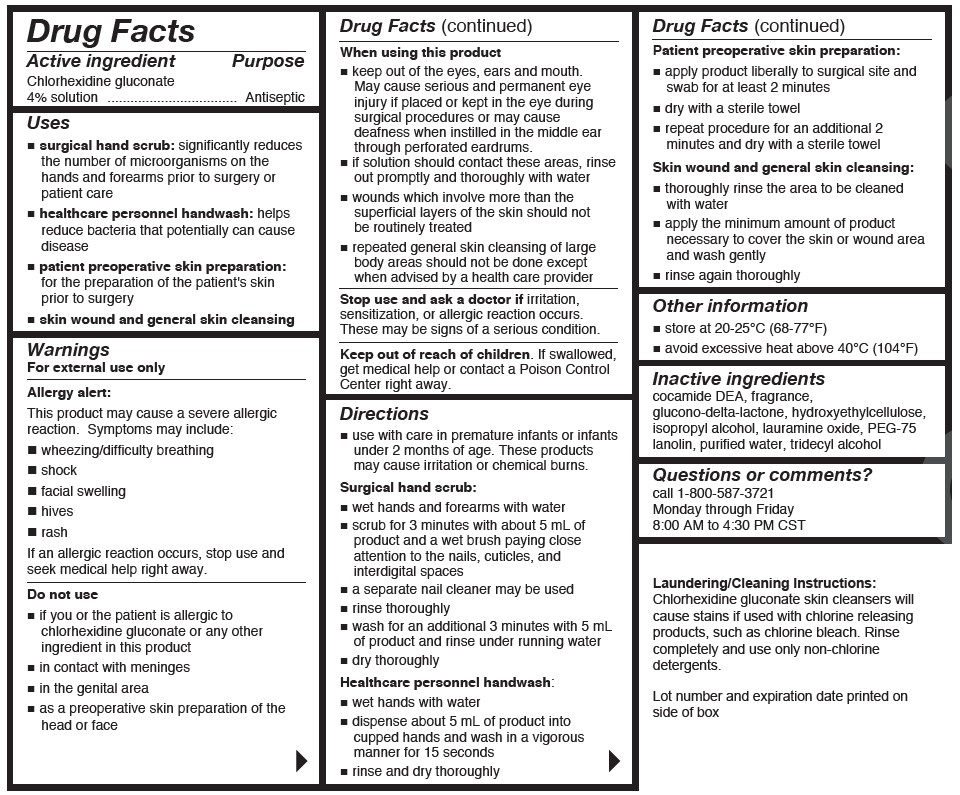
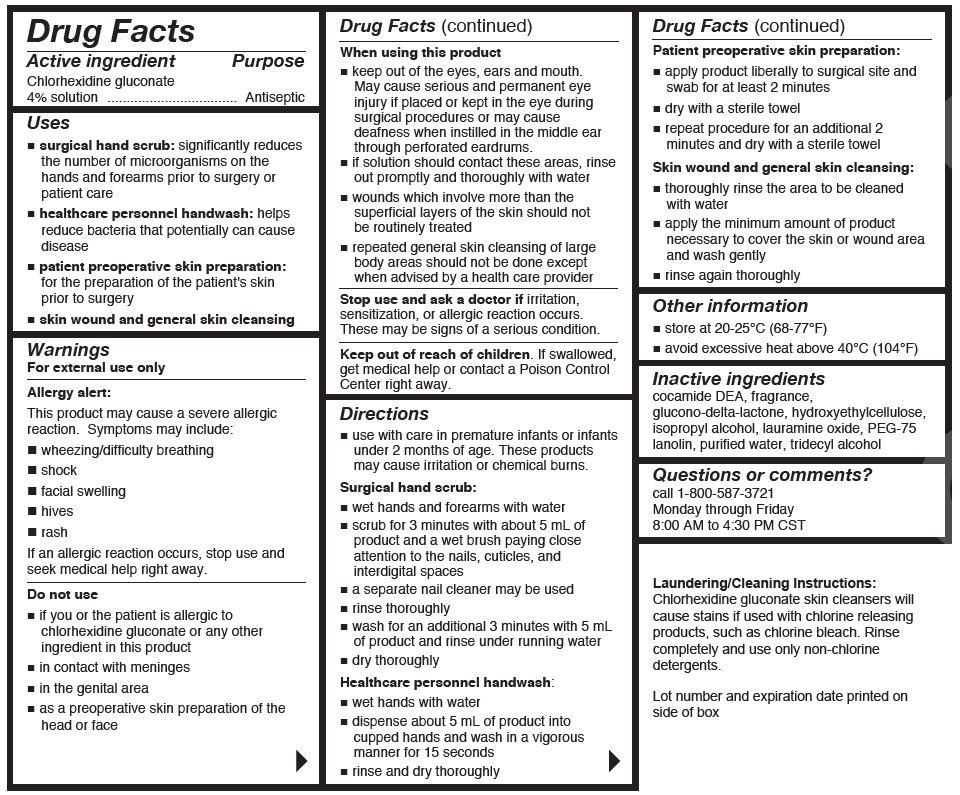
- Active ingredient
- Purpose
-
Uses
- surgical hand scrub: significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
- healthcare personnel handwash: helps reduce bacteria that potentially can cause disease
- patient preoperative skin preparation: for the preparation of the patient's skin prior to surgery
- skin wound and general skin cleansing
-
Warnings
For external use only
Allergy alert:
This product may cause a severe allergic reaction.Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do not use
- if you or the patient is allergic to chlorhexidine gluconate or any other ingredient in this product
- in contact with the meninges
- in the genital area
When using this product
- keep out of eyes, ears and mouth. May cause serious and permanent eye injury if placed or kept in the eye during surgical procedures or may cause deafness when instilled in the middle ear through perforated eardurms.
- if solution should contact these areas, rinse out promptly and thoroughly with water
- wounds which involve more than the superficial layers of the skin should not be routinely treated
- repeated general skin cleansing of large body areas should not be done except when advised by a healthcare provider
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand scrub:
- wet hands and forearms with water
- scrub for 3 minutes with about 5mL of product and a wet brush paying close attention to the nails, cuticles, and interdigital spaces
- a seperate nail cleaner may be used
- rinse thoroughly
- wash for an additional 3 minutes with 5mL of product and rinse under running water
- dry thoroughly
Healthcare personnel handwash:
- wet hands with water
- dispense about 5mL of product into cupped hands and wash in a vigrous manner for 15 seconds
- rinse and dry thoroughly
Patient preoperative skin preparation:
- apply product liberally to surgical site and swab for at least 2 minutes
- dry with a sterile towel
- repeat procedure for an additional 2 minutes and dry with a sterile towel
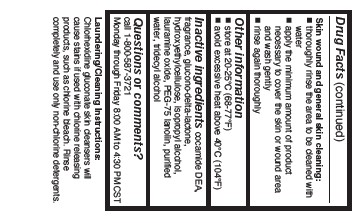
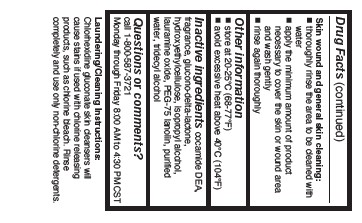
Skin wound and general skin cleansing:
- thoroughly rinse the area to be cleaned with water
- apply the minimum amount of product necessary to cover the skin or wound area and wash gently
- rinse again thoroughly
- Other information
- Inactive ingredients
- Questions or comments?
- Laudering/Cleaning Instructions
-
Display Panels
NDC 69494-901-04
REF 90104
CliniClean
Chlorhexidine Gluconate 4%
Antispetic/Antimicrobial Solution
Non-Sterile
Patient Pre-Operative Prep, Surgical Hand Scrub, Healthcare Personnel Hand Wash. General Skin & Wound Cleanser
For Single-Use
Net Content: 4 fl oz (118mL)
DYE FREE
Manufactured for:
HR Pharmaceuticals, Inc.
York, PA 17402
cliniclean.com
Rev 4170-0 (02/2023)
4HRP04BTLLBL




NDC 69494-901-15
REF 901515
CliniClean
Chlorhexidine Gluconate 4%
Antispetic/Antimicrobial Solution
Non-Sterile
Patient Pre-Operative Prep, Surgical Hand Scrub, Healthcare Personnel Hand Wash. General Skin & Wound Cleanser
For Single-Use
Net Contents: 50 Single-Use 0.5 fl oz (15 mL) Packettes
DYE FREE
Manufactured for:
HR Pharmaceuticals, Inc.
York, PA 17402
cliniclean.com
Rev 4175-0 (02/2023)
4HRP15INNCTN




4HRP15PACKET


-
INGREDIENTS AND APPEARANCE
CLINICLEAN
chlorhexidine gluconate 4% solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69464-901 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength PEG-75 LANOLIN (UNII: 09179OX7TB) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) WATER (UNII: 059QF0KO0R) TRIDECYL ALCOHOL (UNII: 8I9428H868) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISOPROPYL ALCOHOL (UNII: ND2M416302) COCO DIETHANOLAMIDE (UNII: 92005F972D) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69464-901-15 50 in 1 CARTON 03/23/2023 1 15 mL in 1 PACKET; Type 0: Not a Combination Product 2 NDC:69464-901-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/23/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019125 03/23/2023 Labeler - HR Pharmaceuticals, Inc. (117164328) Registrant - Xttrium Laboratories, Inc. (007470579) Establishment Name Address ID/FEI Business Operations Xttrium Laboratories, Inc 007470579 manufacture(69464-901)