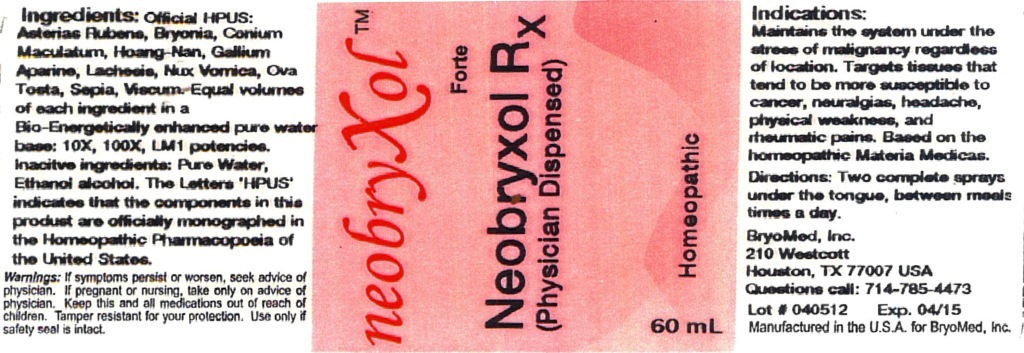
Label: NEOBRYXOL FORTE- asterias rubens, bryonia, conium maculatum, galium aparine, hoang-nan, lachesis mutus, nux vomica, ova tosta, sepia and viscum album liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 45984-0002-1 - Packager: Bryomed Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 5, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Inactive Ingredients
-
Purpose
Indication: maintains the system under the stress of malignancy regardless of location. Targets tissues that tend to be more susceptible to:
- cancer
- neuralgias
- dropsy of extremities
- yellowed skin
- headache
- physical weakness
- rheumatic pains
Reference image forte.jpg
- Dosage and Administration
-
Warnings
Warnings: If symptoms persist or worsen, seek advice of physician. If pregnant or nursing, take only on advice of physician. Keep this and all medication out of reach of children. Tamper resistant for your protection. Use only if safety seal is intact.
Caution: Federal Law prohibits dispensing without prescription.
Reference image forte.jpg - KEEP OUT OF REACH OF CHILDREN
-
Indications and Usage
Indication: Maintains the system under the stress of malignancy regardless of location. Targets tissues that tend to be more susceptible to cancer, neuralgias and dropsy of extremities, yellowed skin, headache, physical weakness and rheumatic pains.
Based on the homeopathic Materia Medicas.
Reference image forte.jpg - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEOBRYXOL FORTE
asterias rubens, bryonia, conium maculatum, galium aparine, hoang-nan, lachesis mutus, nux vomica, ova tosta, sepia and viscum album liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45984-0002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASTERIAS RUBENS (UNII: A7FYY9Q742) (ASTERIAS RUBENS - UNII:A7FYY9Q742) ASTERIAS RUBENS 10 [hp_X] in 30 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 10 [hp_X] in 30 mL CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 10 [hp_X] in 30 mL STRYCHNOS WALLICHIANA BARK (UNII: OQ16ZEE7O7) (STRYCHNOS WALLICHIANA BARK - UNII:OQ16ZEE7O7) STRYCHNOS WALLICHIANA BARK 10 [hp_X] in 30 mL GALIUM APARINE (UNII: Z4B6561488) (GALIUM APARINE - UNII:Z4B6561488) GALIUM APARINE 10 [hp_X] in 30 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 10 [hp_X] in 30 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 10 [hp_X] in 30 mL EGG SHELL, COOKED (UNII: 24HBF856C8) (EGG SHELL, COOKED - UNII:24HBF856C8) EGG SHELL, COOKED 10 [hp_X] in 30 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 10 [hp_X] in 30 mL VISCUM ALBUM WHOLE (UNII: E6839Q6DO1) (VISCUM ALBUM WHOLE - UNII:E6839Q6DO1) VISCUM ALBUM WHOLE 10 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45984-0002-1 30 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/04/2012 Labeler - Bryomed Inc. (078382220) Registrant - Bryomed Inc. (078382220) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture