Label: AMPICILLIN- ampicillin sodium injection, powder, for solution
- NDC Code(s): 0781-9412-15, 0781-9412-92, 0781-9413-15, 0781-9413-92
- Packager: Sandoz Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
For Intravenous Injection Only
Rx only
In ADD-Vantage® Drug Delivery System
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ampicillin and other antibacterial drugs, ampicillin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
DESCRIPTION
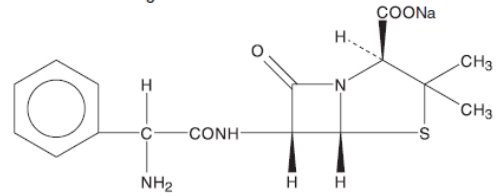
Ampicillin for Injection, USP the monosodium salt of [2S-[2α,5α,6β(S*)]]-6-[(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, is a synthetic penicillin. It is an antibacterial agent with a broad spectrum of bactericidal activity against both penicillin-susceptible Gram-positive organisms and many common Gram-negative pathogens.
Ampicillin for Injection, USP is a white to cream-tinged, crystalline powder. The reconstituted solution is clear, colorless and free from visible particulates.
Each ADD-Vantage® vial of Ampicillin for Injection, USP contains ampicillin sodium equivalent to 1 gram or 2 grams ampicillin. Ampicillin for Injection, USP contains 2.9 milliequivalents of sodium per gram of ampicillin.
It has the following molecular structure:
The molecular formula is C16H18N3NaO4S, and the molecular weight is 371.39. The pH range of the reconstituted solution is 8 to 10.
-
CLINICAL PHARMACOLOGY
Ampicillin for Injection, USP diffuses readily into most body tissues and fluids. However, penetration into the cerebrospinal fluid and brain occurs only when the meninges are inflamed. Ampicillin is excreted largely unchanged in the urine and its excretion can be delayed by concurrent administration of probenecid. Due to maturational changes in renal function, ampicillin half-life decreases as postmenstrual age (a sum of gestational age and postnatal age) increases for infants with postnatal age of less than 28 days. The active form appears in the bile in higher concentrations than those found in serum. Ampicillin is the least serum-bound of all the penicillins, averaging about 20% compared to approximately 60 to 90% for other penicillins. Ampicillin for Injection, USP is well tolerated by most patients and has been given in doses of 2 grams daily for many weeks without adverse reactions.
Microbiology
While in vitro studies have demonstrated the susceptibility of most strains of the following organisms, clinical efficacy for infections other than those included in the INDICATIONS AND USAGE section has not been demonstrated.
Antibacterial Activity
The following bacteria have been shown in in vitro studies to be susceptible to Ampicillin for Injection, USP:
Gram-positive Bacteria
Hemolytic and nonhemolytic streptococci
Streptococcus pneumoniae
Nonpenicillinase-producing staphylococci
Clostridium spp.
B. anthracis
Listeria monocytogenes
Most strains of enterococci.
Gram-negative Bacteria
H. influenzae
N. gonorrhoeae
N. meningitidis
Proteus mirabilis
Many strains of Salmonella, Shigella, and E. coli.
AMPICILLIN does not resist destruction by penicillinase.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
INDICATIONS AND USAGE
Ampicillin for Injection, USP is indicated in the treatment of infections caused by susceptible strains of the designated organisms in the following conditions:
Respiratory Tract Infections caused by Streptococcus pneumoniae. Staphylococcus aureus (penicillinase and nonpenicillinase-producing), H. influenzae, and Group A beta-hemolytic Streptococci.
Bacterial Meningitis caused by E. coli, Group B Streptococci, and other Gram-negative bacteria (Listeria monocytogenes, N. meningitidis). The addition of an aminoglycoside with ampicillin may increase its effectiveness against Gram-negative bacteria.
Septicemia and Endocarditis caused by susceptible Gram-positive organisms including Streptococcus spp., penicillin G-susceptible staphylococci, and enterococci. Gram-negative sepsis caused by E. coli, Proteus mirabilis and Salmonella spp. responds to ampicillin. Endocarditis due to enterococcal strains usually respond to intravenous therapy. The addition of an aminoglycoside may enhance the effectiveness of ampicillin when treating streptococcal endocarditis.
Urinary Tract Infections caused by sensitive strains of E. coli and Proteus mirabilis.
Gastrointestinal Infections caused by Salmonella typhi (typhoid fever), other Salmonella spp., and Shigella spp. (dysentery) usually respond to oral or intravenous therapy.
Bacteriology studies to determine the causative organisms and their susceptibility to ampicillin should be performed. Therapy may be instituted prior to obtaining results of susceptibility testing. It is advisable to reserve the parenteral form of this drug for moderately severe and severe infections and for patients who are unable to take the oral forms. A change to oral ampicillin may be made as soon as appropriate.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ampicillin for Injection, USP and other antibacterial drugs, Ampicillin for Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Indicated surgical procedures should be performed.
- CONTRAINDICATIONS
-
WARNINGS
Serious and occasionally fatal hypersensitivity (anaphylactoid) reactions have been reported in patients on penicillin therapy. Although anaphylaxis is more frequent following parenteral therapy, it has occurred in patients on oral penicillins. These reactions are more apt to occur in individuals with a history of penicillin hypersensitivity and/or a history of sensitivity to multiple allergens.
There have been well-documented reports of individuals with a history of penicillin hypersensitivity reactions who have experienced severe hypersensitivity reactions when treated with a cephalosporin. Before initiating therapy with a penicillin, careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins, and other allergens. If an allergic reaction occurs, the drug should be discontinued and appropriate therapy instituted.
SERIOUS ANAPHYLACTOID REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE, OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ampicillin for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
The possibility of superinfections with mycotic organisms or bacterial pathogens should be kept in mind during therapy. In such cases, discontinue the drug and substitute appropriate treatment.
A high percentage (43 to 100 percent) of patients with infectious mononucleosis who receive ampicillin develop a skin rash. Typically, the rash appears 7 to 10 days after the start of oral ampicillin therapy and remains for a few days to a week after the drug is discontinued. In most cases, the rash is maculopapular, pruritic, and generalized. Therefore, the administration of ampicillin is not recommended in patients with mononucleosis. It is not known whether these patients are truly allergic to ampicillin.
Prescribing Ampicillin for Injection, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be counseled that antibacterial drugs including ampicillin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When ampicillin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ampicillin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Laboratory Tests
As with any potent drug, periodic assessment of organ system function, including renal, hepatic, and hematopoietic, should be made during prolonged therapy.
Transient elevation of serum transaminase has been observed following administration of ampicillin. The significance of this finding is not known.
Drug Interactions
The concurrent administration of allopurinol and ampicillin increases substantially the incidence of skin rashes in patients receiving both drugs as compared to patients receiving ampicillin alone. It is not known whether this potentiation of ampicillin rashes is due to allopurinol or the hyperuricemia present in these patients.
Drug/Laboratory Test Interactions
With high urine concentrations of ampicillin, false-positive glucose reactions may occur if Clinitest, Benedict’s Solution, or Fehling’s Solution are used. Therefore, it is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as Clinistix or Tes-Tape) be used.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
No long-term animal studies have been conducted with this drug.
Pregnancy
Reproduction studies have been performed in laboratory animals at doses several times the human dose and have revealed no evidence of adverse effects due to ampicillin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery
Oral ampicillin-class antibiotics are poorly absorbed during labor. Studies in guinea pig showed that intravenous administration of ampicillin slightly decreased the uterine tone and frequency of contractions, but moderately increased the height and duration of contractions. However, it is not known whether use of these drugs in humans during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor, or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary.
Nursing Mothers
Ampicillin is excreted in trace amounts in human milk. Therefore, caution should be exercised when ampicillin-class antibiotics are administered to a nursing woman.
Pediatric Use
Guidelines for the administration of these drugs to children, including neonates are presented in DOSAGE AND ADMINISTRATION section.
-
ADVERSE REACTIONS
As with other penicillins, it may be expected that untoward reactions will be essentially limited to sensitivity phenomena. They are more likely to occur in individuals who have previously demonstrated hypersensitivity to penicillins and in those with a history of allergy, asthma, hay fever, or urticaria.
The following adverse reactions have been reported as associated with the use of ampicillin:
Gastrointestinal
Glossitis, stomatitis, black “hairy” tongue, nausea, vomiting, enterocolitis, pseudomembranous colitis, and diarrhea. (These reactions are usually associated with oral dosage forms.)
Hypersensitivity Reactions
Skin rashes and urticaria have been reported frequently. A few cases of exfoliative dermatitis and erythema multiforme have been reported. Linear IgA bullous dermatosis has been reported. Anaphylaxis is the most serious reaction experienced and has usually been associated with the parenteral dosage form.
Note: Urticaria, other skin rashes, and serum sickness-like reactions may be controlled with antihistamines and, if necessary, systemic corticosteroids. Whenever such reactions occur, ampicillin should be discontinued, unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to ampicillin therapy. Serious anaphylactic reactions require the immediate use of epinephrine, oxygen, and intravenous steroids.
Liver– A moderate rise in serum glutamic oxaloacetic transaminase (SGOT) has been noted, particularly in infants, but the significance of this finding is unknown. Mild transitory SGOT elevations have been observed in individuals receiving larger (two to four times) than usual and oft-repeated intramuscular injections. Evidence indicates that glutamic oxaloacetic transaminase (GOT) is released at the site of intramuscular injection of ampicillin sodium and that the presence of increased amounts of this enzyme in the blood does not necessarily indicate liver involvement.
Hemic and Lymphatic Systems– Anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia, and agranulocytosis have been reported during therapy with the penicillins. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena.
Central Nervous System - Seizures
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
The Single Use ADD-Vantage® vials are for intravenous use only.
Infections of the respiratory tract and soft tissues.
Patients weighing 40 kg (88 lbs) or more: 250 mg to 500 mg every 6 hours.
Patients weighing less than 40 kg (88 lbs): 25 to 50 mg/kg/day in equally divided doses at 6- to 8- hour intervals.
Infections of the gastrointestinal and genitourinary tracts (including those caused by Neisseria gonorrhoeae in females).
Patients weighing 40 kg (88 lbs) or more: 500 mg every 6 hours.
Patients weighing less than 40 kg (88 lbs): 50 mg/kg/day in equally divided doses at 6- to 8- hour intervals.
In the treatment of chronic urinary tract and intestinal infections, frequent bacteriological and clinical appraisal is necessary. Smaller doses than those recommended above should not be used. Higher doses should be used for stubborn or severe infections. In stubborn infections, therapy may be required for several weeks. It may be necessary to continue clinical and/or bacteriological follow-up for several months after cessation of therapy.
Urethritis in males due to N. gonorrhoeae.
Adults – Two doses of 500 mg each at an interval of 8 to 12 hours. Treatment may be repeated if necessary or extended if required.
In the treatment of complications of gonorrheal urethritis, such as prostatitis and epididymitis, prolonged and intensive therapy is recommended. Cases of gonorrhea with a suspected primary lesion of syphilis should have darkfield examinations before receiving treatment. In all other cases where concomitant syphilis is suspected, monthly serological tests should be made for a minimum of four months.
The doses for the preceding infections may be given by either the intramuscular or intravenous route. A change to oral ampicillin may be made when appropriate.
Bacterial Meningitis
Adults and children – 150 to 200 mg/kg/day in equally divided doses every 3 to 4 hours. (Treatment may be initiated with intravenous drip therapy and continued with intramuscular injections.) The doses for other infections may be given by either the intravenous or intramuscular route.
Neonates (less than or equal to 28 days of postnatal age) - Dosage should be based on Gestational age and Postnatal age according to Table 3.
Table 3 Dosage in Neonates (less than or equal to 28 days of postnatal age) for Bacterial Meningitis and Septicemia
Gestational age (weeks)
Postnatal age (days)
Dosage
less than or equal to 34
- less than or equal to 7
- 100 mg/kg/day in equally divided doses every 12 hours
less than or equal to 34
- greater than or equal to 8 and less than 28
- 150 mg/kg/day in equally divided doses every 12 hours
greater than 34
- less than or equal to 28
- 150 mg/kg/day in equally divided doses every 8 hours
Septicemia
Adults and children – 150 to 200 mg/kg/day. Start with intravenous administration for at least three days and continue with the intramuscular route every 3 to 4 hours.
Neonates (less than or equal to 28 days of postnatal age) - Dosage should be based on Gestational age and Postnatal age according to Table 3 (above).
Treatment of all infections should be continued for a minimum of 48 to 72 hours beyond the time that the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. A minimum of 10 days treatment is recommended for any infection caused by Group A beta-hemolytic streptococci to help prevent the occurrence of acute rheumatic fever or acute glomerulonephritis.
-
DIRECTIONS FOR USE
ADD-Vantage® Vial – ampicillin for injection 1 and 2 grams.
The ADD-Vantage® vials for intravenous use only are to be used with ADD-Vantage® diluent containers: 0.9% Sodium Chloride Injection, USP, 50 mL and 100 mL, or 5% Dextrose Injection, USP, 50 mL and 100 mL.
Reconstitute ADD-Vantage® vial as directed in INSTRUCTIONS FOR USE.
Use only freshly prepared solutions. Intravenous injections should be administered immediately after reconstitution since the potency may decrease.
Summary of Utility Times for Constituted Ampicillin for Injection in the Hospira ADD-Vantage® System Vial Size
IV Solution
Final
Concentration
Utility Times
1 g Vial
5% Dextrose Injection, USP
10 mg/mL
2 Hours at 25°C
1 g Vial
0.9% Sodium Chloride Injection, USP
10 mg/mL
8 Hours at 25°C
2 g Vial
5% Dextrose Injection, USP
20 mg/mL
1 Hour at 25°C
2 g Vial
0.9% Sodium Chloride Injection, USP
40 mg/mL
6 Hours at 25°C
-
INSTRUCTIONS FOR USE
To Open Diluent Container:
Peel overwrap from the corner and remove container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible Diluent Container:
(Use Aseptic Technique)
- 1.
- Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
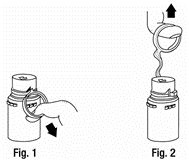
a. To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (SEE FIGURE 1), then pull straight up to remove the cap. (SEE FIGURE 2.)
NOTE: Do not access vial with syringe.
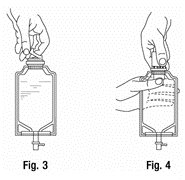
b. To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (SEE FIGURE 3.) - 2.
- Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately 1/2 turn (180°) after the first audible click. (SEE FIGURE 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go. NOTE: Once vial is seated, do not attempt to remove. (SEE FIGURE 4.)
- 3.
- Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
- 4.
- Label appropriately.
To Prepare Admixture:
- 1.
- Squeeze the bottom of the diluent container gently to inflate the portion of the container surrounding the end of the drug vial.
- 2.
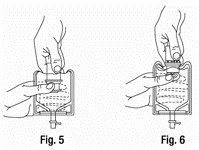
- With the other hand, push the drug vial down into the container telescoping the walls of the container. Grasp the inner cap of the vial through the walls of the container. (SEE FIGURE 5.)
- 3.
- Pull the inner cap from the drug vial. (SEE FIGURE 6.) Verify that the rubber stopper has been pulled out, allowing the drug and diluent to mix.
- 4.
- Mix container contents thoroughly and use within the specified time.
Preparation for Administration:
(Use Aseptic Technique)
- 1.
- Confirm the activation and admixture of vial contents.
- 2.
- Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
- 3.
- Close flow control clamp of administration set.
- 4.
- Remove cover from outlet port at bottom of container.
- 5.
- Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated.
NOTE: See full directions on administration set carton. - 6.
- Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
- 7.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- 8.
- Open flow control clamp and clear air from set. Close clamp.
- 9.
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- 10.
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
-
HOW SUPPLIED
Ampicillin for Injection, USP ADD-Vantage® for intravenous injection.
Ampicillin sodium equivalent to 1 or 2 grams ampicillin per vial.
NDC 0781-9412-92 1 gram ADD-Vantage® Vial, packed in 10s
NDC 0781-9413-92 2 grams ADD-Vantage® Vial, packed in 10s
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Clinitest is a registered trademark of Miles, Inc.
Clinistix is a registered trademark of Bayer Corporation.
Tes-Tape is a registered trademark of Eli Lilly Company.
ADD-Vantage® is a trademark of Hospira Inc.
Novaplus is a registered trademarks of Vizient, Inc.
Manufactured by Sandoz GmbH
for Sandoz Inc., Princeton, NJ 08540
Rev. January 2024
- 1 gram Label
- 2 grams Label
-
INGREDIENTS AND APPEARANCE
AMPICILLIN
ampicillin sodium injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0781-9412 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMPICILLIN SODIUM (UNII: JFN36L5S8K) (AMPICILLIN - UNII:7C782967RD) AMPICILLIN 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0781-9412-92 10 in 1 PACKAGE 02/19/1987 1 NDC:0781-9412-15 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA062738 02/19/1987 AMPICILLIN
ampicillin sodium injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0781-9413 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMPICILLIN SODIUM (UNII: JFN36L5S8K) (AMPICILLIN - UNII:7C782967RD) AMPICILLIN 2 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0781-9413-92 10 in 1 PACKAGE 02/19/1987 1 NDC:0781-9413-15 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA062738 02/19/1987 Labeler - Sandoz Inc (005387188)