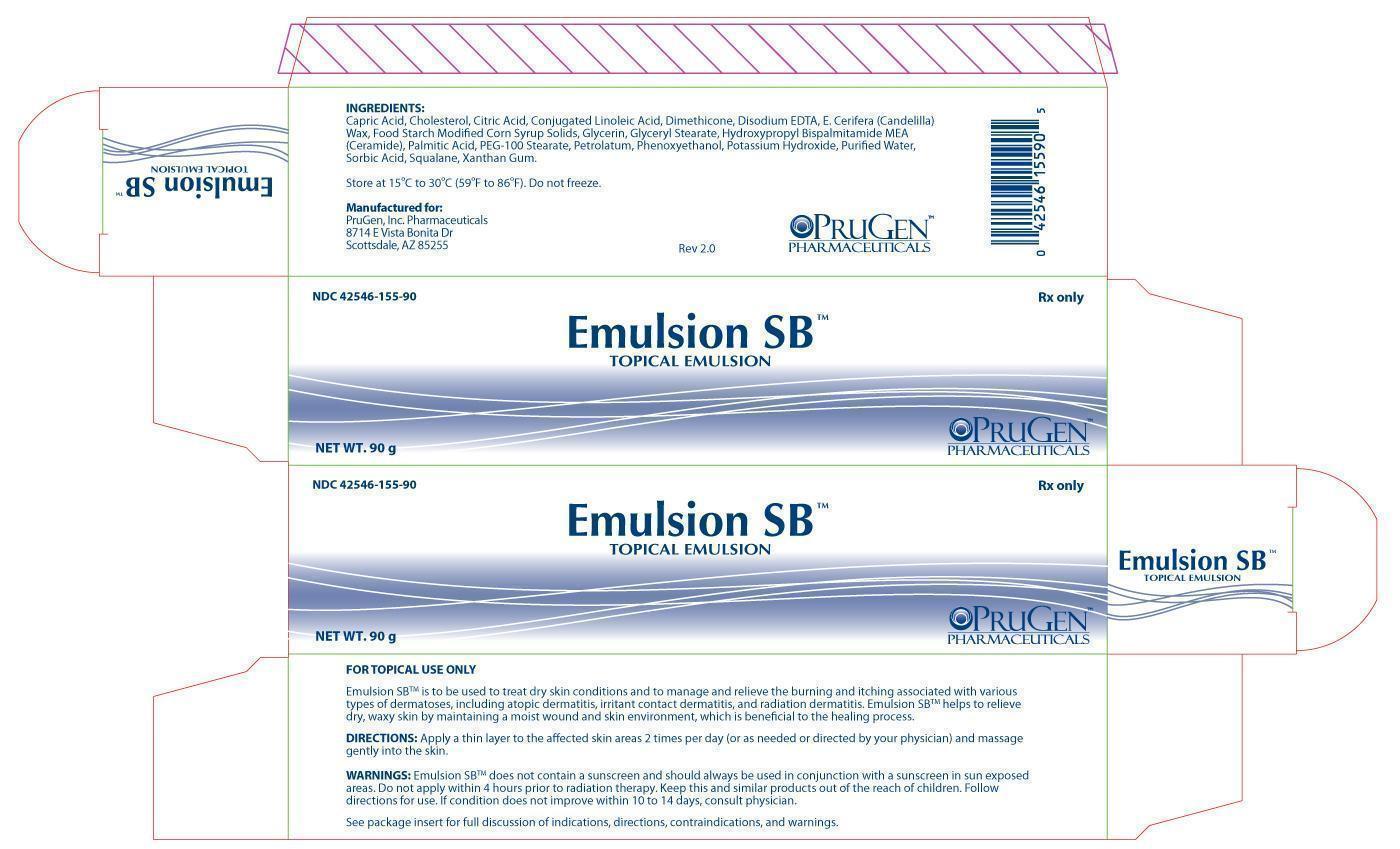
Label: EMULSION SB-
- NHRIC Code(s): 42546-155-90, 42546-155-50, 42546-155-52
- Packager: PruGen, Inc.
- Category: PRESCRIPTION MEDICAL DEVICE LABEL
- DEA Schedule: None
- Marketing Status: Premarket Notification
Drug Label Information
Updated August 29, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
INDICATIONS & USAGE
Emulsion SB is to be used to treat dry skin conditions and to manage and relieve the burning and itching associated with various types of dermatoses, including atopic dermatitis, irritant contact dermatitis, and radiation dermatitis. Emulsion SB helps to relieve dry, waxy skin by maintaining a moist wound and skin environment, which is beneficial to the healing process.
- CONTRAINDICATIONS
-
WARNINGS
• Emulsion SB does not contain a sunscreen and should always be used in conjunction with a sunscreen in sun exposed areas
• Do not apply within 4 hours prior to radiation therapy
• Keep this and similar products out of the reach of children
• Follow directions for use
• If condition does not improve within 10 to 14 days, consult physician
-
PRECAUTIONS AND OBSERVATIONS
For the treatment of any dermal wound, consult a physician.
• Use Emulsion SB only as directed
• Emulsion SB is for external use only and should not be ingested or taken internally
• If clinical signs of infection are present, appropriate treatment should be initiated. If clinically indicated, use of Emulsion SB may be continued during the anti-infective therapy
• In radiation dermatitis and/or in conjunction with ongoing radiation therapy, apply following radiation therapy or as indicated by the radiation therapist
- INSTRUCTIONS FOR USE
-
INGREDIENTS
Capric Acid, Cholesterol, Citric Acid, Conjugated Linoleic Acid, Dimethicone, Disodium EDTA, E. Cerifera (Candelilla) Wax, Food Starch Modified Corn Syrup Solids, Glycerin, Glyceryl Stearate, Hydroxypropyl Bispalmitamide MEA (Ceramide), Palmitic Acid, PEG-100 Stearate, Petrolatum, Phenoxyethanol, Potassium Hydroxide, Purified Water, Sorbic Acid, Squalane, Xanthan Gum.
-
HOW SUPPLIED
Emulsion SB is supplied as follows:
NDC 42546-155-90: 90 gram tube
NDC 42546-155-50: 50 gram tube
NDC 42546-155-52: Package contains two 50 gram tubes
Store at 15ºC to 30ºC (59ºF to 86ºF). Do not freeze.
Rx ONLY - Prescription Medical Device; Federal Law restricts this device to sale by or on the order of a physician.
Manufactured for:
PruGen, Inc. Pharmaceuticals
8714 E Vista Bonita Dr
Scottsdale, AZ 85255
- Emulsion SB Carton Label
-
INGREDIENTS AND APPEARANCE
EMULSION SB
dressing, wound, drugProduct Information Product Type PRESCRIPTION MEDICAL DEVICE Item Code (Source) NHRIC:42546-155 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:42546-155-90 1 in 1 BOX 1 90 g in 1 TUBE 2 NHRIC:42546-155-50 1 in 1 BOX 2 50 g in 1 TUBE 3 NHRIC:42546-155-52 2 in 1 BOX 3 50 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K110732 05/05/2011 Labeler - PruGen, Inc. (929922750)