Label: EXCHANGE SELECT- vegetable laxative from senna concentrate tablet
- NDC Code(s): 55301-107-00
- Packager: ARMY AND AIR FORCE EXCHANGE SERVICE
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- take preferably at bedtime or as directed by a doctor
age
starting dosage
maximum dosage
adults and children
12 years of age and over2 tablets once a day
4 tablets twice a day
children 6 to under 12 years
1 tablet once a day
2 tablets twice a day
children 2 to under 6 years
1/2 tablet once a day
1 tablet twice a day
children under 2 years
ask a doctor
ask a doctor
- SPL UNCLASSIFIED SECTION
- INACTIVE INGREDIENT
-
TAMPER EVIDENT: Do not use if printed seal under cap is broken or missing
IMPORTANT: Keep this carton for future reference on full labeling.
*This product is not manufactured or distributed by Avrio Health L.P., owner of the registered trademark Senokot®
“ SATISFACTION GUARANTEED OR YOUR MONEY BACK”
Manufactured For Your Military Exchanges By: Raritan Pharmaceuticals Inc., 8 Joanna Court, East Brunswick, NJ 08816 -
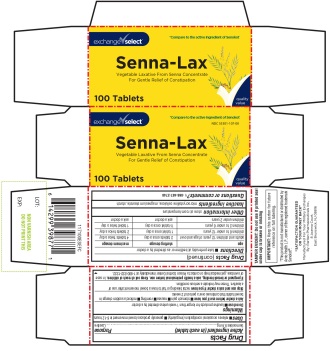
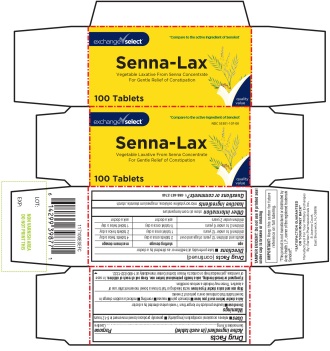
PRINCIPAL DISPLAY PANEL
*Compare to the active ingredient of Senokot®
exchange√select
Senna-Lax
Vegetable Laxative From Senna Concentrate For Gentle Relief of Constipation
100 Tablets
*Compare to the active ingredient of Senokot®
NDC 55301-107-00
exchange√select
Senna-Lax
Vegetable Laxative From Senna Concentrate For Gentle Relief of Constipation
100 Tablets
-
INGREDIENTS AND APPEARANCE
EXCHANGE SELECT
vegetable laxative from senna concentrate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55301-107 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor Imprint Code RP117 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55301-107-00 1 in 1 CARTON 02/09/2021 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 02/09/2021 Labeler - ARMY AND AIR FORCE EXCHANGE SERVICE (001695568)