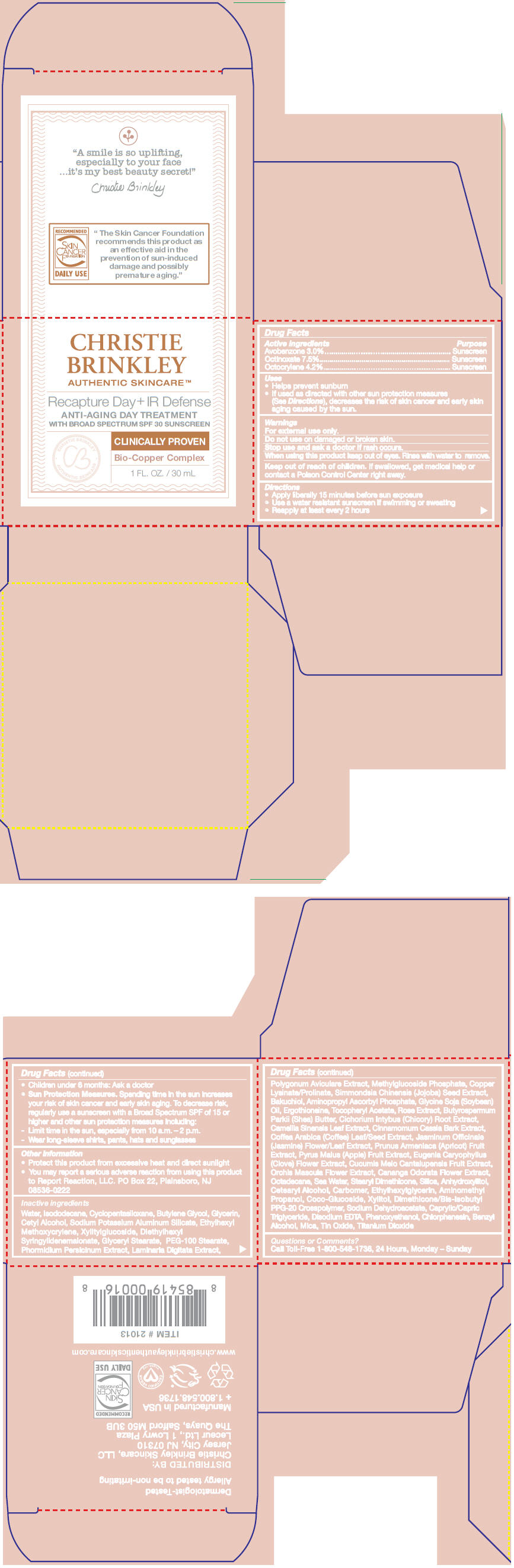
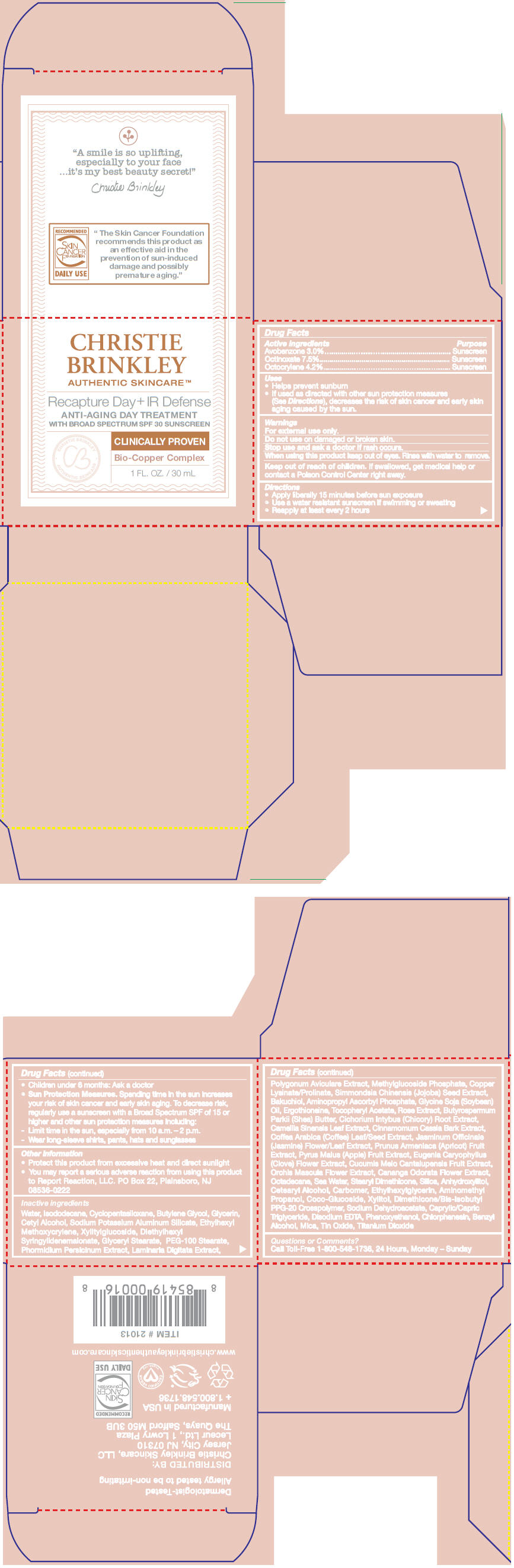
Label: RECAPTURE DAY PLUS IR DEFENSE- avobenzone, octocrylene, and octinoxate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70550-000-07, 70550-000-68, 70550-000-83, 70550-000-98 - Packager: Atlantic Coast Media Group, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 24, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (See Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. – 2 p.m.
- Wear long-sleeve shirts, pants, hats and sunglasses
- Other information
-
Inactive ingredients
Water, Isododecane, Cyclopentasiloxane, Butylene Glycol, Glycerin, Cetyl Alcohol, Sodium Potassium Aluminum Silicate, Ethylhexyl Methoxycrylene, Xylitylglucoside, Diethylhexyl Syringylidenemalonate, Glyceryl Stearate, PEG-100 Stearate, Phormidium Persicinum Extract, Laminaria Digitata Extract, Polygonum Aviculare Extract, Methylglucoside Phosphate, Copper Lysinate/Prolinate, Simmondsia Chinensis (Jojoba) Seed Extract, Bakuchiol, Aminopropyl Ascorbyl Phosphate, Glycine Soja (Soybean) Oil, Ergothioneine, Tocopheryl Acetate, Rose Extract, Butyrospermum Parkii (Shea) Butter, Cichorium Intybus (Chicory) Root Extract, Camellia Sinensis Leaf Extract, Cinnamomum Cassia Bark Extract, Coffea Arabica (Coffee) Leaf/Seed Extract, Jasminum Officinale (Jasmine) Flower/Leaf Extract, Prunus Armeniaca (Apricot) Fruit Extract, Pyrus Malus (Apple) Fruit Extract, Eugenia Caryophyllus (Clove) Flower Extract, Cucumis Melo Cantalupensis Fruit Extract, Orchis Mascula Flower Extract, Cananga Odorata Flower Extract, Octadecane, Sea Water, Stearyl Dimethicone, Silica, Anhydroxylitol, Cetearyl Alcohol, Carbomer, Ethylhexylglycerin, Aminomethyl Propanol, Coco-Glucoside, Xylitol, Dimethicone/Bis-Isobutyl PPG-20 Crosspolymer, Sodium Dehydroacetate, Caprylic/Capric Triglyceride, Disodium EDTA, Phenoxyethanol, Chlorphenesin, Benzyl Alcohol, Mica, Tin Oxide, Titanium Dioxide
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Jar Carton
-
INGREDIENTS AND APPEARANCE
RECAPTURE DAY PLUS IR DEFENSE
avobenzone, octocrylene, and octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70550-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 42 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) XYLITYLGLUCOSIDE (UNII: O0IEZ166FB) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) PHORMIDIUM PERSICINUM (UNII: ZA983U4810) LAMINARIA DIGITATA (UNII: 15E7C67EE8) POLYGONUM AVICULARE TOP (UNII: ZCD6009IUF) SIMMONDSIA CHINENSIS SEED (UNII: D24K2Q1F6H) BAKUCHIOL (UNII: OT12HJU3AR) AMINOPROPYL ASCORBYL PHOSPHATE (UNII: 290O2PQ83R) SOYBEAN OIL (UNII: 241ATL177A) ERGOTHIONEINE (UNII: BDZ3DQM98W) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SHEA BUTTER (UNII: K49155WL9Y) CHICORY ROOT (UNII: 090CTY533N) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CHINESE CINNAMON (UNII: WS4CQ062KM) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) APRICOT (UNII: 269CJD5GZ9) APPLE (UNII: B423VGH5S9) CLOVE (UNII: K48IKT5321) CANTALOUPE (UNII: 8QF5D5H6UH) ORCHIS MASCULA FLOWER (UNII: 6H1JQK35LA) CANANGA ODORATA FLOWER (UNII: 76GTF6Z97M) OCTADECANE (UNII: N102P6HAIU) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARYL DIMETHICONE (400 MPA.S AT 50C) (UNII: R327X197HY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ANHYDROXYLITOL (UNII: 8XWR7NN42F) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) COCO GLUCOSIDE (UNII: ICS790225B) XYLITOL (UNII: VCQ006KQ1E) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) EDETATE DISODIUM (UNII: 7FLD91C86K) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) BENZYL ALCOHOL (UNII: LKG8494WBH) MICA (UNII: V8A1AW0880) STANNIC OXIDE (UNII: KM7N50LOS6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70550-000-68 1 in 1 CARTON 03/01/2015 1 30 mL in 1 JAR; Type 0: Not a Combination Product 2 NDC:70550-000-98 1 in 1 CARTON 03/01/2015 2 30 mL in 1 JAR; Type 0: Not a Combination Product 3 NDC:70550-000-83 1 in 1 CARTON 03/01/2015 3 15 mL in 1 JAR; Type 0: Not a Combination Product 4 NDC:70550-000-07 1 in 1 CARTON 03/01/2015 4 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/01/2015 Labeler - Atlantic Coast Media Group, LLC. (800337946)