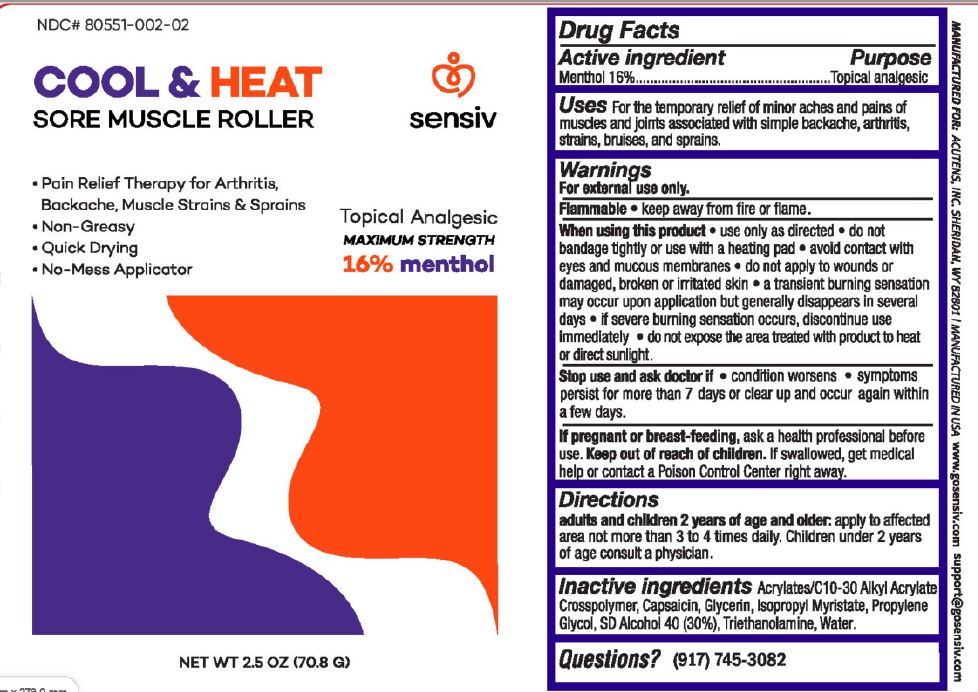
Label: COOL AND HEAT SORE MUSCLE ROLLER- menthol gel
- NDC Code(s): 80551-002-02
- Packager: Acutens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients Purpose
- PURPOSE
- Uses:
-
Warnings
For external use only
Flammable: Keep away from fire or flame.
When using this product• use only as directed • do not bandage tightly or use with a heating pad • avoid contact with eyes and mucous membranes • do not apply to wounds or damaged, broken or irritated skin • a transient burning sensation may occur upon application but generally disappears in several days • if severe burning sensation occurs, discontinue use immediately • do not expose the area treated with product to heat or direct sunlight.
Stop use and ask doctor if• condition worsens •symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
- Direction
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COOL AND HEAT SORE MUSCLE ROLLER
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80551-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 16 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) CAPSAICIN (UNII: S07O44R1ZM) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80551-002-02 70.8 g in 1 CONTAINER; Type 0: Not a Combination Product 03/10/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/10/2021 Labeler - Acutens (051133165)