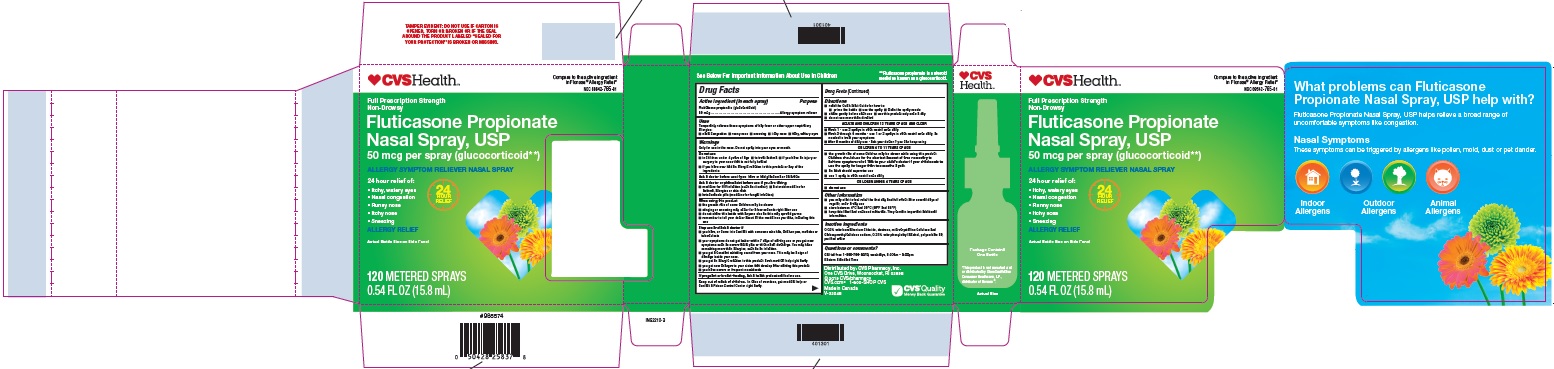
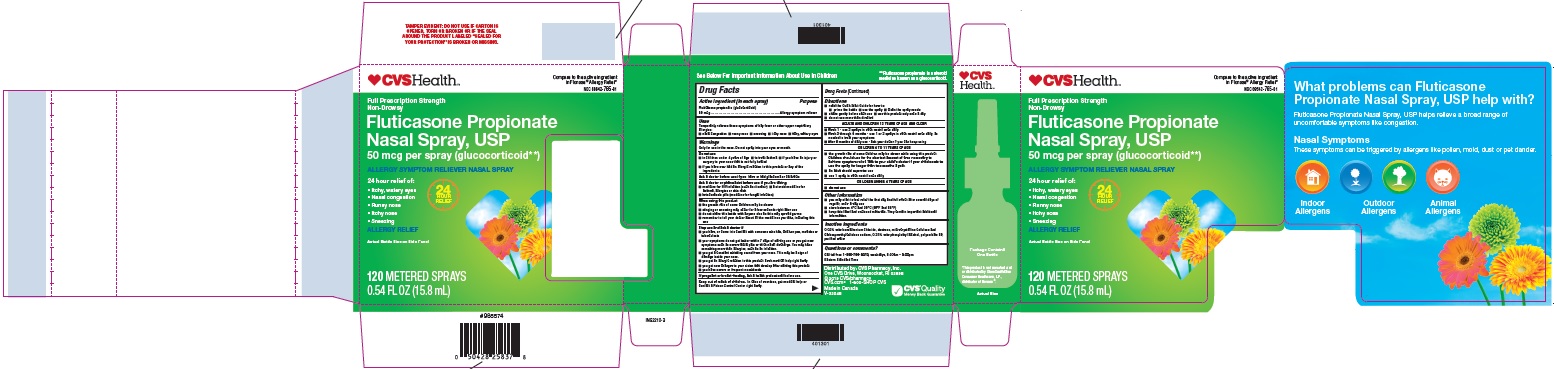
Label: FLUTICASONE PROPIONATE spray, metered
- NDC Code(s): 69842-765-01, 69842-765-02, 69842-765-03, 69842-765-04
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each spray)
- Purpose
- Uses
-
Warnings
Only for use in the nose. Do not spray into your eyes or mouth.
Do not use
- in children under 4 years of age
- to treat asthma
- if you have an injury or surgery to your nose that is not fully healed
- if you have ever had an allergic reaction to this product or any of the ingredients
Ask a doctor or pharmacist before use if you are taking
- medicine for HIV infection (such as ritonavir)
- a steroid medicine for asthma, allergies or skin rash
- ketoconazole pills (medicine for fungal infection)
When using this product
- the growth rate of some children may be slower
- stinging or sneezing may occur for a few seconds right after use
- do not share this bottle with anyone else as this may spread germs
- remember to tell your doctor about all the medicines you take, including this one
Stop use and ask a doctor if
- you have, or come into contact with someone who has, chicken pox, measles or tuberculosis
- your symptoms do not get better within 7 days of starting use or you get new symptoms such as severe facial pain or thick nasal discharge. You may have something more than allergies, such as an infection.
- you get a constant whistling sound from your nose. This may be a sign of damage inside your nose.
- you get an allergic reaction to this product. Seek medical help right away.
- you get new changes to your vision that develop after starting this product
- you have severe or frequent nosebleeds
-
Directions
- read the Quick Start Guide for how to:
- prime the bottle
- use the spray
- clean the spray nozzle
- shake gently before each use
- use this product only once a day
- do not use more than directed
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER
-
Week 1 - use 2 sprays in each nostril once daily
-
Week 2 through 6 months - use 1 or 2 sprays in each nostril once daily, as needed to treat your symptoms
-
After 6 months of daily use - ask your doctor if you can keep using
CHILDREN 4 TO 11 YEARS OF AGE
-
the growth rate of some children may be slower while using this product. Children should use for the shortest amount of time necessary to achieve symptom relief. Talk to your child's doctor if your child needs to use the spray for longer than two months a year.
-
an adult should supervise use
-
use 1 spray in each nostril once daily
CHILDREN UNDER 4 YEARS OF AGE
-
do not use
- read the Quick Start Guide for how to:
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel - Carton 120md (single)
- Principal Display Panel - Bottle 120md
- Principal Display Panel - Carton 60md
- Principal Display Panel - Bottle 60 md
-
INGREDIENTS AND APPEARANCE
FLUTICASONE PROPIONATE
fluticasone propionate spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-765 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUTICASONE PROPIONATE (UNII: O2GMZ0LF5W) (Fluticasone - UNII:CUT2W21N7U) FLUTICASONE PROPIONATE 50 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-765-01 1 in 1 CARTON 09/12/2019 1 120 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:69842-765-02 2 in 1 CARTON 09/12/2019 2 120 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:69842-765-03 3 in 1 CARTON 09/12/2019 02/28/2025 3 120 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC:69842-765-04 1 in 1 CARTON 09/12/2019 02/28/2025 4 60 in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208150 09/12/2019 Labeler - CVS Pharmacy (062312574) Registrant - Apotex Inc. (209429182) Establishment Name Address ID/FEI Business Operations Apotex Inc. 255092496 analysis(69842-765) , manufacture(69842-765) Establishment Name Address ID/FEI Business Operations Legacy Pharmaceutical Packaging, LLC 143213275 pack(69842-765)