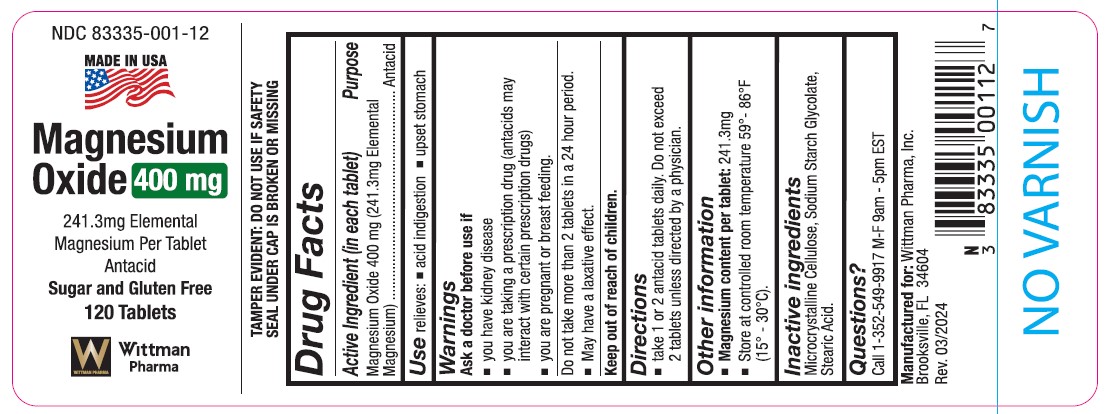
Label: MAGNESIUM OXIDE tablet
- NDC Code(s): 83335-001-12
- Packager: Wittman Pharma, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- WARNINGS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- ACTIVE INGREDIENT
-
Drug Facts
ACTIVE INGREDIENT (In each tablet)
Magnesium Oxide 400mg (241.3mg elemental magnesium)
Purpose
Antacid
USES
relieves - acid indigestion - upset stomach
WARNINGS
Ask a doctor before use if:
You have kidney disease
You are taking a prescription drug (antacids may interact with certain prescription drugs).
You are pregnant or breast feeding.
Do not take more than 2 tablets in a 24 hour period.
May have a laxative effect.
Keep out of reach of children.
DIRECTIONS
take one or two antacid tablets daily. Do not exceed two tablets unless directed by a physician.
OTHER INFORMATION
Magnesium content per tablet: 241.3mg
store at room temperature 59°- 86° F (15°- 30°C)INACTIVE INGREDIENTS
microcrystalline cellulose, sodium starch, glyocolate, stearic acid
Questions?
1-352-549-9917 M-F 9am - 5pm EST
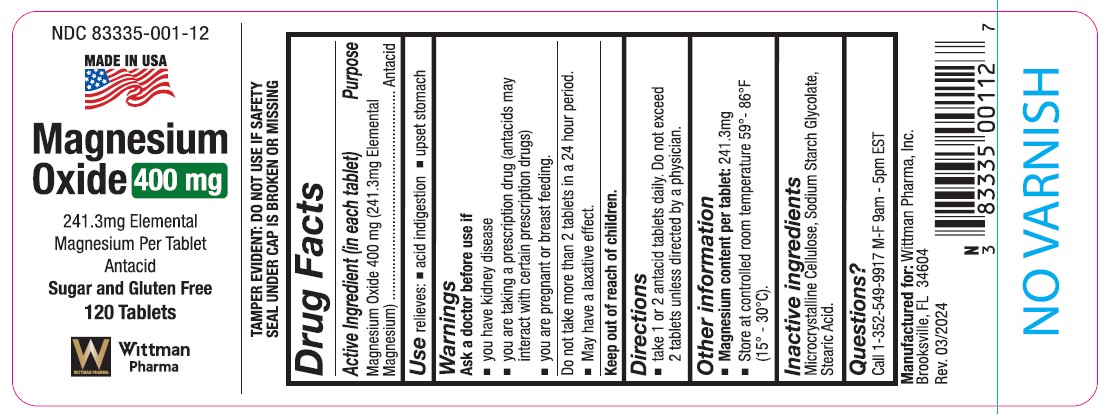
-
INGREDIENTS AND APPEARANCE
MAGNESIUM OXIDE
magnesium oxide tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83335-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 241.3 mg in 1000 mg Inactive Ingredients Ingredient Name Strength SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white (White to Off-White) Score score with uneven pieces Shape ROUND (Tablet) Size 4mm Flavor Imprint Code MG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83335-001-12 120 mg in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/16/2023 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 03/16/2023 Labeler - Wittman Pharma, Inc. (830980947) Registrant - Wittman Pharma, Inc. (830980947) Establishment Name Address ID/FEI Business Operations Wittman Pharma, Inc. 830980947 manufacture(83335-001) , analysis(83335-001) , label(83335-001)