Label: DUKAL POVIDONE-IODINE SCRUB SPONGE STICKS- povidone-iodine scrub sponge sticks sponge
- NDC Code(s): 65517-0040-1
- Packager: Dukal LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings:
- Directions
- Other Information:
- Inactive Ingredients:
-
PRINCIPAL DISPLAY PANEL
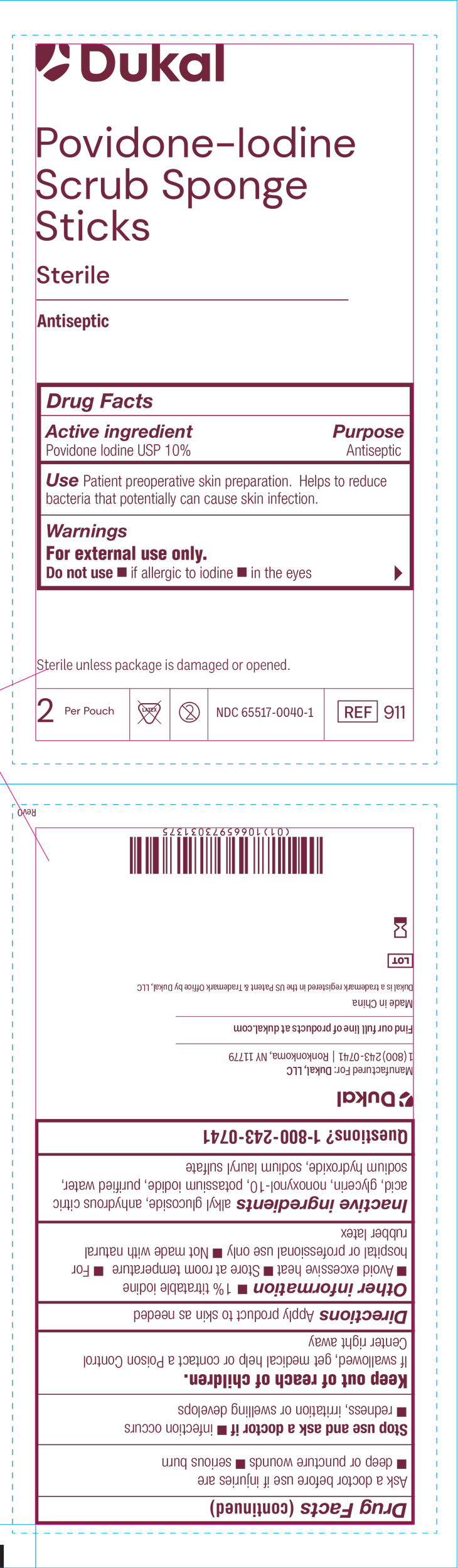 Dukal™
Dukal™
Povidone-Iodine
Scrub Sponge
Sticks
Sterile
Antispetic
Drug Facts
Active ingredient Purpose
Povidone Iodine USP 10% Antiseptic
Use Patient preoperative skin preparation. Helps to reduce bacteria
that potentially can cause skin infection.
Warnings
For external use only.
Do no use if allergic to iodine, in the eyes
Sterile unless package is damagedor opened
2 Per Pouch Not Made with Natural Rubber Latex Do not re-use NDC-66517-0040-1 REF 911
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DUKAL POVIDONE-IODINE SCRUB SPONGE STICKS
povidone-iodine scrub sponge sticks spongeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65517-0040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 1.1 g in 100 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POTASSIUM IODIDE (UNII: 1C4QK22F9J) GLYCERIN (UNII: PDC6A3C0OX) NONOXYNOL-10 (UNII: K7O76887AP) WATER (UNII: 059QF0KO0R) C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-0040-1 2 in 1 POUCH 03/15/2023 1 72 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 03/15/2023 Labeler - Dukal LLC (791014871)



