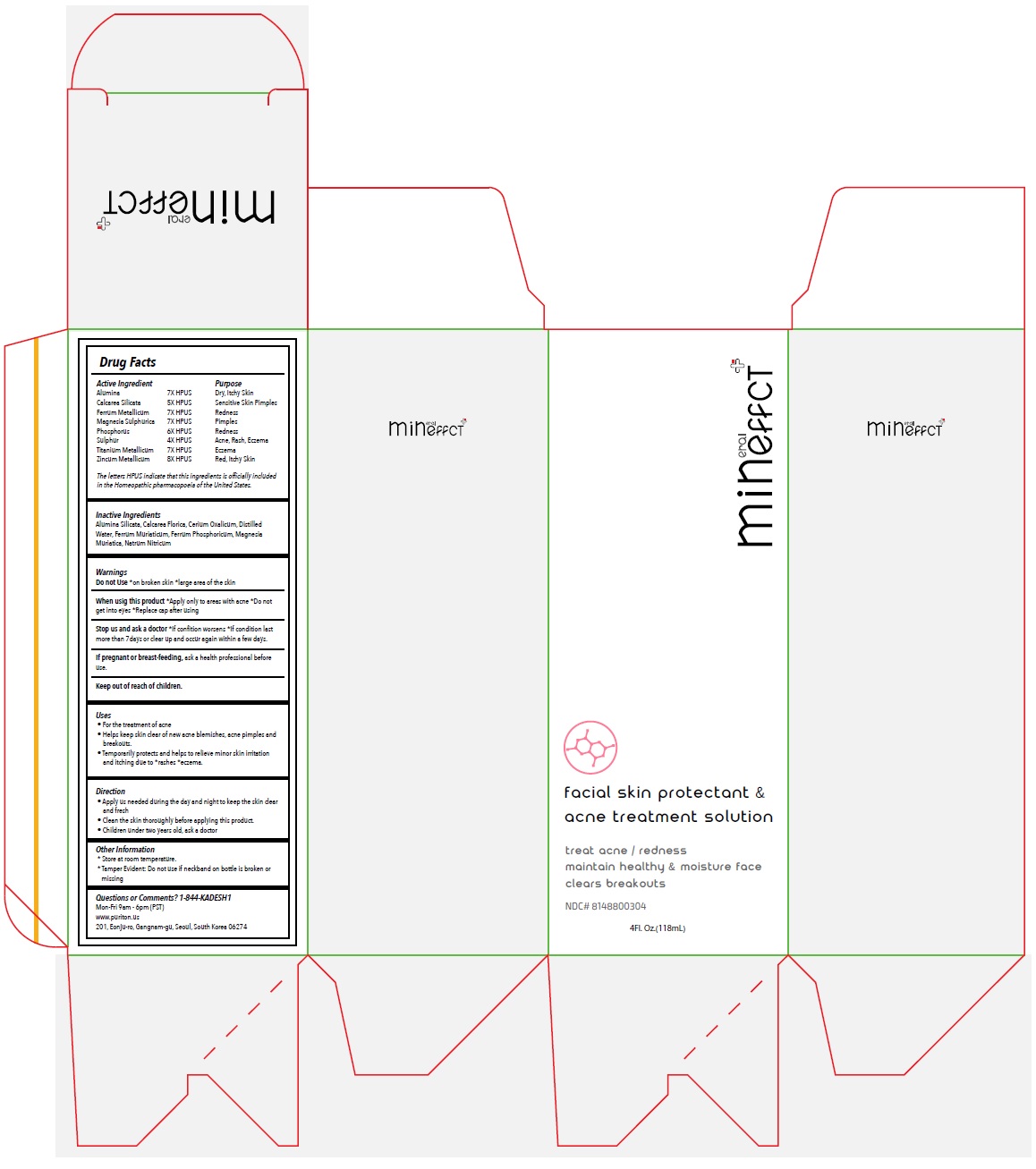
Label: MINEFFECT FACIAL SKIN PROTECTANT AND ACNE TREATMENT SOLUTION- alumina, calcarea silicata, ferrum metallicum, magnesia sulphurica, phosphorus, sulphur, titanium metallicum, zincum metallicum spray
- NDC Code(s): 81488-003-04
- Packager: Kadesh Incoporation Co,Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Active Ingredient
Alumina 7X HPUS
Calcarea Silicata 5X HPUS
Ferrum Metallicum 7X HPUS
Magnesia Sulphurica 7X HPUS
Phosphorus 6X HPUS
Sulphur 4X HPUS
Titanium Metallicum 7X HPUS
Zincum Metallicum 8X HPUSThe letters HPUS indicate that this ingredients is officially included in the Homeopathic pharmacopoeia of the United States.
- Purpose
- Inactive Ingredients
-
Warnings
Do not Use*on broken skin *large area of the skin
When using this product*Apply only to areas with acne *Do not get into eyes *Replace cap after using
Stop use and ask a doctor*If condition worsens *If condition last more than 7days or clear up and occur again within a few days.
If pregnant or breast-feeding, ask a health professional before use. - Uses
- Direction
- Other Information
- Questions or Comments? 1-844-KADESH1
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
MINEFFECT FACIAL SKIN PROTECTANT AND ACNE TREATMENT SOLUTION
alumina, calcarea silicata, ferrum metallicum, magnesia sulphurica, phosphorus, sulphur, titanium metallicum, zincum metallicum sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81488-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM OXIDE (UNII: LMI26O6933) (ALUMINUM OXIDE - UNII:LMI26O6933) ALUMINUM OXIDE 7 [hp_X] in 118 mL CALCIUM SILICATE (UNII: S4255P4G5M) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SILICATE 5 [hp_X] in 118 mL IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 7 [hp_X] in 118 mL MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE HEPTAHYDRATE 7 [hp_X] in 118 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 6 [hp_X] in 118 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 4 [hp_X] in 118 mL TITANIUM (UNII: D1JT611TNE) (TITANIUM - UNII:D1JT611TNE) TITANIUM 7 [hp_X] in 118 mL ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 8 [hp_X] in 118 mL Inactive Ingredients Ingredient Name Strength KAOLIN (UNII: 24H4NWX5CO) CALCIUM FLUORIDE (UNII: O3B55K4YKI) CEROUS OXALATE NONAHYDRATE (UNII: 0UV74P3R0J) WATER (UNII: 059QF0KO0R) FERRIC CHLORIDE HEXAHYDRATE (UNII: 0I2XIN602U) FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM NITRATE (UNII: 8M4L3H2ZVZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81488-003-04 1 in 1 BOX 02/01/2021 1 118 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/01/2021 Labeler - Kadesh Incoporation Co,Ltd (694615354) Establishment Name Address ID/FEI Business Operations Kadesh Incoporation Co,Ltd 694615354 manufacture(81488-003)