Label: JOCK ITCH ANTIFUNGAL- clotrimazole cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 29500-2434-1 - Packager: Personal Care Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 23, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
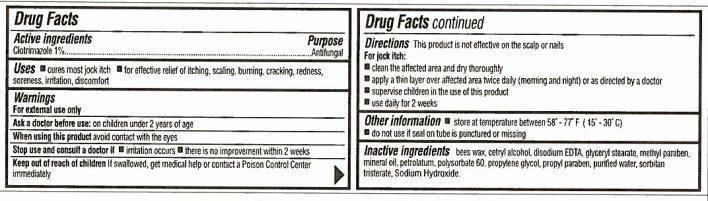
- Active ingredients
- Purpose
- Keep out of reach of children
- Uses
- Warnings
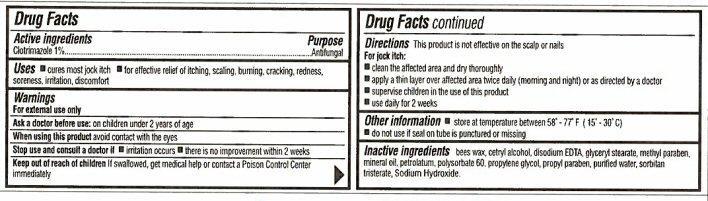
- Directions
- Other information
- Inactive ingredients
-
Product Label
DRUGSTORE-Rx
SMART WELLNESS
Clotrimazole USP 1%
Jock Itch
Antifungal Cream
Cures most types of jock itch fungus
Relieves
- Itching
- Burning
- Scaling
- Cracking
Greaseless
Odorless
Non-staining
NET WT. 1.5 OZ. (42 g)
DRUGSTORE-Rx
SMART WELLNESS delivers outstanding quality in healthcare products, at truly amazing prices. We employ the highest standard when searching the world to bring the best value to your doorstep. Use our products confidently, knowing that attention and care has gone into every item. That's smart wellness!
Distributed by: PERSONAL CARE PRODUCTS, LLC
Troy, Michigan 48084 U.S.A.
Code No. MH/DRUGS/KD-313 Made in India
-
INGREDIENTS AND APPEARANCE
JOCK ITCH ANTIFUNGAL
clotrimazole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29500-2434 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 420 mg in 42 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ALCOHOL (UNII: 936JST6JCN) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) POLYSORBATE 60 (UNII: CAL22UVI4M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITAN TRISTEARATE (UNII: 6LUM696811) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29500-2434-1 42 g in 1 TUBE; Type 0: Not a Combination Product 07/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 07/10/2013 Labeler - Personal Care Products, Inc. (966155082) Registrant - Personal Care Products, Inc. (966155082) Establishment Name Address ID/FEI Business Operations Anicare Pharmaceuticals Pvt. Ltd. 916837425 manufacture(29500-2434)