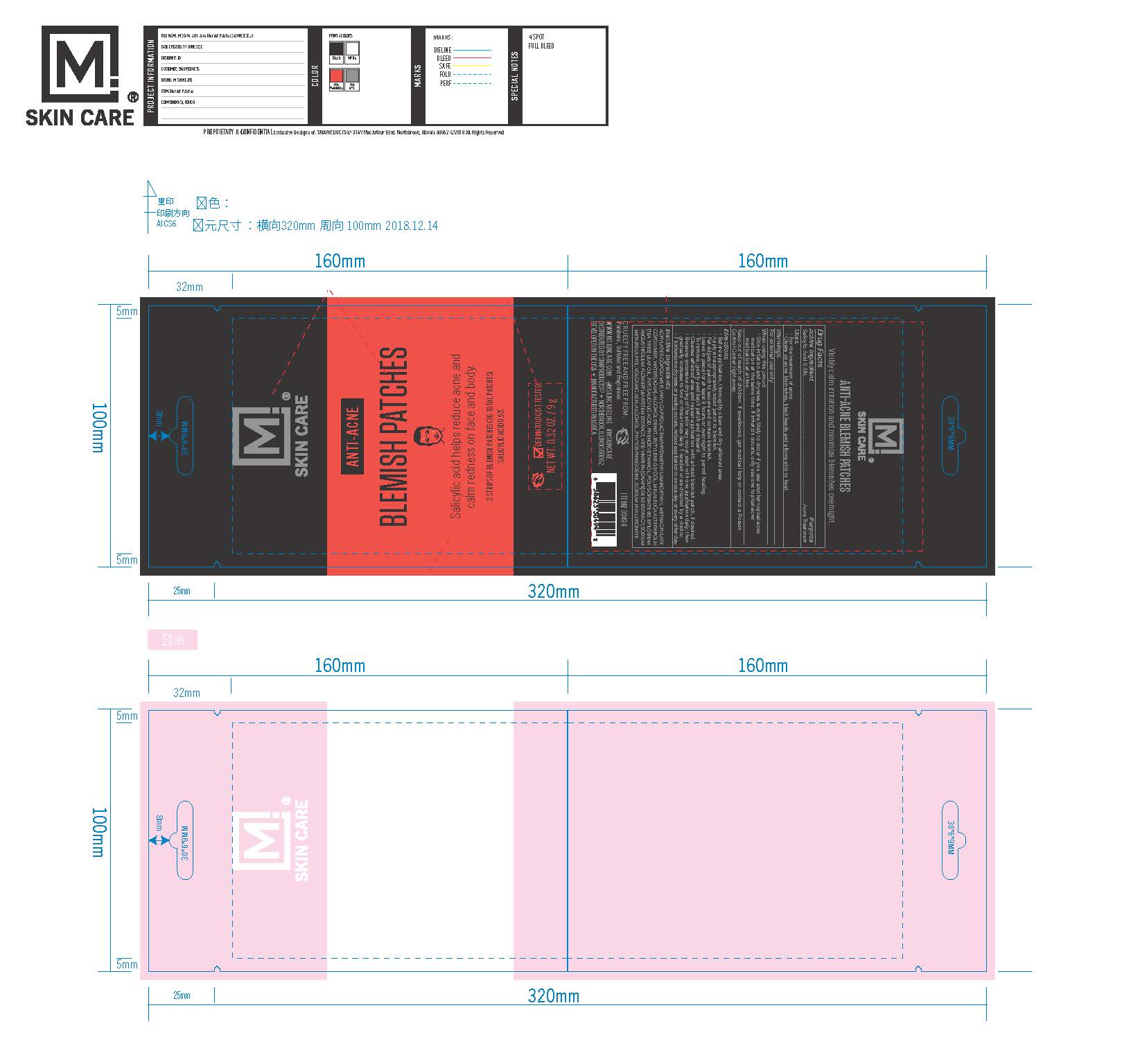
Label: M SKIN CARE BLEMISH PATCHES- salicylic acid 0.5% patch
- NDC Code(s): 71420-102-09
- Packager: Sales and Product Solutions
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- When using this product
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Before application, thoroughly clean and dry affected area.
Apply an appropriately sized patch to blemish
Pat edges of patch to secure and contain blemish.
Leave in place for at least 8 hours or overnight to permit healing
To remove, gently peel back patch and discard.
Cleanse affected area and repeat application with a fresh blemish patch, if desired.
Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
If bothersome dryness or peeling occurs, reduce application to once a day or every other day -
INACTIVE INGREDIENT
Acrylates Copolymer,Vinyl Caprolactam/VP/Dimethylaminoethyl Methacrylate Copolymer, water, Alcohol Denat. Butylene Glycol, Melaleuca Alternifolia (Tea Tree) Leaf Oil,PVP, Salicylic Acid, Phenoxyethanol, Polysorbate 80, Epilobium Angustifolium Flower/Leaf/Stem Extract, Vitis Vinifera (Grape) Seed Extract, Sodium Metabisulfite, Volcanic Ash, Alcohol, Phytosphingosine, Sodium Hyaluronate.
- M Skin Care Blemish Patches
-
INGREDIENTS AND APPEARANCE
M SKIN CARE BLEMISH PATCHES
salicylic acid 0.5% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71420-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 g Inactive Ingredients Ingredient Name Strength BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) STREPTOCOCCUS PYOGENES (UNII: LJ2LP0YL98) ALCOHOL (UNII: 3K9958V90M) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) 2-PROPENOIC ACID, 2-(PHOSPHONOOXY)- (UNII: 545YL308OW) PEG-9 DIGLYCIDYL ETHER/SODIUM HYALURONATE CROSSPOLYMER (UNII: 788QAG3W8A) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) EPILOBIUM ANGUSTIFOLIUM LEAF (UNII: 7NV86426N2) VITIS VINIFERA SEED (UNII: C34U15ICXA) SODIUM METABISULFITE (UNII: 4VON5FNS3C) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71420-102-09 1 in 1 POUCH; Type 0: Not a Combination Product 07/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 07/01/2020 Labeler - Sales and Product Solutions (037519852)