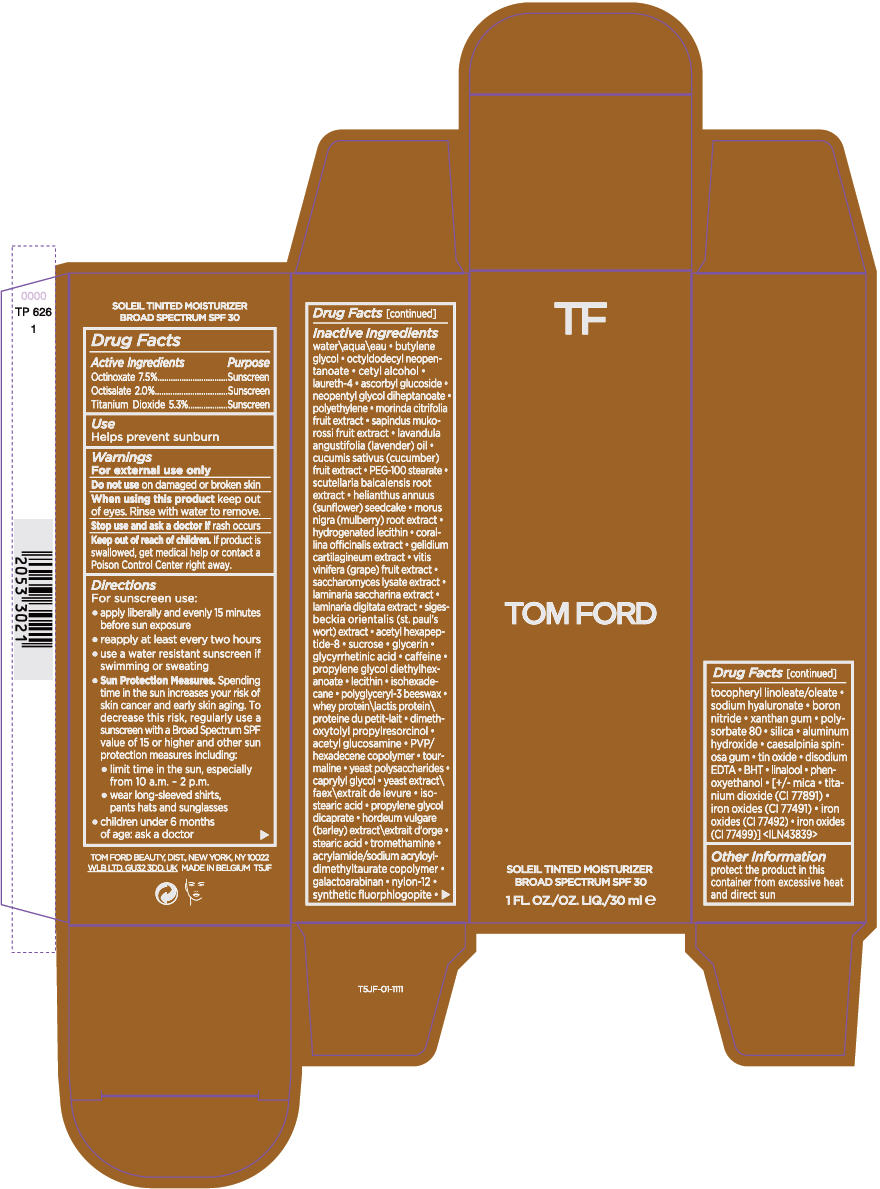
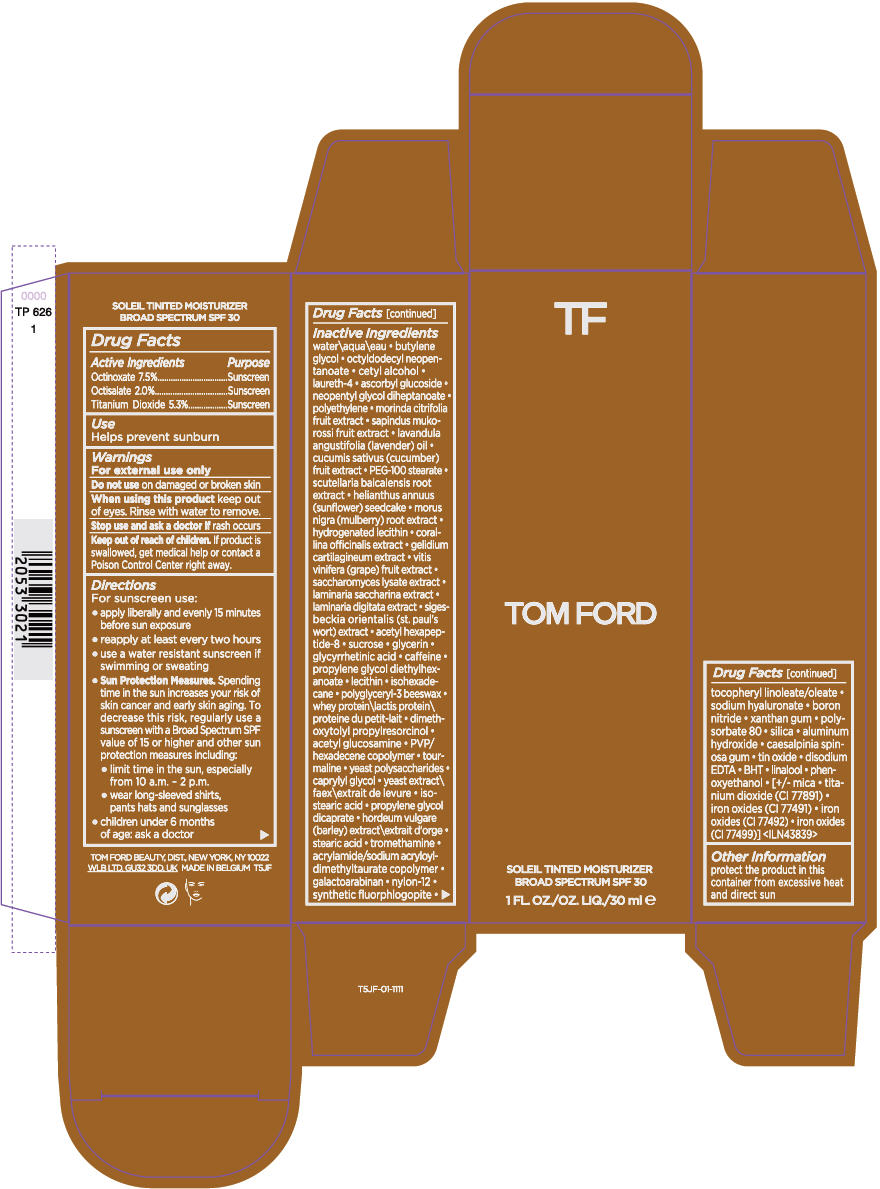
Label: TOM FORD SOLEIL TINTED MOISTURIZER BROAD SPECTRUM SPF 30- octinoxate, octisalate, and titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 76398-005-01 - Packager: TOM FORD BEAUTY DIST
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 11, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive Ingredients
water\aqua\eau • butylene glycol • octyldodecyl neopentanoate • cetyl alcohol • laureth-4 • ascorbyl glucoside • neopentyl glycol diheptanoate • polyethylene • morinda citrifolia fruit extract • sapindus mukorossi fruit extract • lavandula angustifolia (lavender) oil • cucumis sativus (cucumber) fruit extract • PEG-100 stearate • scutellaria baicalensis root extract • helianthus annuus (sunflower) seedcake • morus nigra (mulberry) root extract • hydrogenated lecithin • corallina officinalis extract • gelidium cartilagineum extract • vitis vinifera (grape) fruit extract • saccharomyces lysate extract • laminaria saccharina extract • laminaria digitata extract • sigesbeckia orientalis (st. paul's wort) extract • acetyl hexapeptide-8 • sucrose • glycerin • glycyrrhetinic acid • caffeine • propylene glycol diethylhexanoate • lecithin • isohexadecane • polyglyceryl-3 beeswax • whey protein\lactis protein\proteine du petit-lait • dimethoxytolyl propylresorcinol • acetyl glucosamine • PVP/hexadecene copolymer • tourmaline • yeast polysaccharides • caprylyl glycol • yeast extract\faex\extrait de levure • isostearic acid • propylene glycol dicaprate • hordeum vulgare (barley) extract\extrait d'orge • stearic acid • tromethamine • acrylamide/sodium acryloyldimethyltaurate copolymer • galactoarabinan • nylon-12 • synthetic fluorphlogopite • tocopheryl linoleate/oleate • sodium hyaluronate • boron nitride • xanthan gum • polysorbate 80 • silica • aluminum hydroxide • caesalpinia spinosa gum • tin oxide • disodium EDTA • BHT • linalool • phenoxyethanol • [+/- mica • titanium dioxide (CI 77891) • iron oxides (CI 77491) • iron oxides (CI 77492) • iron oxides (CI 77499)] <ILN43839>
- Other Information
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
TOM FORD SOLEIL TINTED MOISTURIZER BROAD SPECTRUM SPF 30
octinoxate, octisalate, and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76398-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.0825 g in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.022 g in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.0583 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) CETYL ALCOHOL (UNII: 936JST6JCN) LAURETH-4 (UNII: 6HQ855798J) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) NONI FRUIT (UNII: 7829X3G2X5) SAPINDUS MUKOROSSI FRUIT (UNII: 66H9NW427Y) LAVENDER OIL (UNII: ZBP1YXW0H8) CUCUMBER (UNII: YY7C30VXJT) PEG-100 STEARATE (UNII: YD01N1999R) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) HELIANTHUS ANNUUS SEEDCAKE (UNII: 482WYF7XLC) MORUS NIGRA ROOT (UNII: 033456LFG1) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) CORALLINA OFFICINALIS (UNII: 4004498D06) GELIDIUM CARTILAGINEUM (UNII: 37LBZ0E1UE) WINE GRAPE (UNII: 3GOV20705G) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) LAMINARIA DIGITATA (UNII: 15E7C67EE8) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) SUCROSE (UNII: C151H8M554) GLYCERIN (UNII: PDC6A3C0OX) ENOXOLONE (UNII: P540XA09DR) CAFFEINE (UNII: 3G6A5W338E) PROPYLENE GLYCOL DIETHYLHEXANOATE (UNII: 8D8I9Z0F1Z) ISOHEXADECANE (UNII: 918X1OUF1E) DIMETHOXYTOLYL PROPYLRESORCINOL (UNII: 4OD5GIH1UK) N-ACETYLGLUCOSAMINE (UNII: V956696549) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISOSTEARIC ACID (UNII: X33R8U0062) PROPYLENE GLYCOL DICAPRATE (UNII: U783H9JHWY) STEARIC ACID (UNII: 4ELV7Z65AP) TROMETHAMINE (UNII: 023C2WHX2V) GALACTOARABINAN (UNII: SL4SX1O487) NYLON-12 (UNII: 446U8J075B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) BORON NITRIDE (UNII: 2U4T60A6YD) XANTHAN GUM (UNII: TTV12P4NEE) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CAESALPINIA SPINOSA RESIN (UNII: WL3883U2PO) STANNIC OXIDE (UNII: KM7N50LOS6) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) LINALOOL, (+/-)- (UNII: D81QY6I88E) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76398-005-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/01/2017 Labeler - TOM FORD BEAUTY DIST (005914387) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COMPANY, THE 828534516 RELABEL(76398-005) , REPACK(76398-005) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(76398-005) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 202952982 MANUFACTURE(76398-005) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(76398-005) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(76398-005) Establishment Name Address ID/FEI Business Operations NORTHTEC INC 943871157 RELABEL(76398-005) , REPACK(76398-005) Establishment Name Address ID/FEI Business Operations NORTHTEC LLC 959338336 MANUFACTURE(76398-005) Establishment Name Address ID/FEI Business Operations PADC 1 949264774 RELABEL(76398-005) , REPACK(76398-005) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(76398-005) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 255175580 MANUFACTURE(76398-005)