Label: ARSENIC TRIOXIDE injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 54879-027-10, 54879-027-11 - Packager: STI Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 17, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
Arsenic trioxide injection, for intravenous administration
These highlights do not include all the information needed to use Arsenic Trioxide safely and effectively. See full prescribing information for Arsenic Trioxide.
Initial U.S. Approval: 2000WARNING: DIFFERENTIATION SYNDROME AND CARDIAC CONDUCTION ABNORMALITIES
See full prescribing information for complete boxed warning.
- Patients treated with arsenic trioxide injection may develop differentiation syndrome, which can be fatal. If symptoms occur, initiate high-dose steroids immediately and monitor hemodynamics. ( 5.1)
- Arsenic trioxide injection can cause QT interval prolongation and ventricular arrhythmia, which can be fatal. Before administering arsenic trioxide injection, assess the QT interval, correct electrolyte abnormalities, and consider discontinuing drugs known to prolong QT interval. Do not administer arsenic trioxide injection to patients with ventricular arrhythmia or prolonged QTcF. ( 2.3, 5.2)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Arsenic trioxide injection is an arsenical indicated:
- For induction of remission and consolidation in patients with APL who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression. ( 1.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injectable solution for intravenous administration supplied as 10 mg/10 ml of arsenic trioxide in single-dose vials. ( 3)
CONTRAINDICATIONS
Hypersensitivity to arsenic. ( 4)
WARNINGS AND PRECAUTIONS
- Hepatotoxicity: Monitor hepatic function tests at least twice weekly during arsenic trioxide injection therapy. ( 5.3)
- Carcinogenesis: Arsenic trioxide is a human carcinogen. Monitor patients for the development of second primary malignancies. ( 5.4)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise of potential risk to a fetus and use of effective contraception. ( 5.5, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (greater than 30%) were leukocytosis, neutropenia, thrombocytopenia, nausea, vomiting, diarrhea, abdominal pain, hepatic toxicity, fever, rigors, fatigue, insomnia, tachycardia, QTc prolongation, edema, hyperglycemia, hypokalemia, hypomagnesemia, dyspnea, cough, rash or itching, sore throat, arthralgia, headaches, paresthesia, and dizziness. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact STI Pharma LLC, Inc. at 1-888-301-9680 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Lactation: Advise women not to breastfeed. ( 8.2)
- Renal Impairment: Monitor patients with severe renal impairment (creatinine clearance less than 30 mL/min) for toxicity when treated with arsenic trioxide injection; dose reduction may be warranted. ( 8.6)
- Hepatic Impairment: Monitor patients with severe hepatic impairment (Child-Pugh Class C) for toxicity when treated with arsenic trioxide injection. ( 8.7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
1.2 Relapsed or Refractory APL
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dose Modifications for Toxicities
2.3 Instructions for Preparation and Intravenous Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Differentiation Syndrome
5.2 Cardiac Conduction Abnormalities
5.3 Hepatotoxicity
5.4 Carcinogenesis
5.5 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Renal Impairment
8.7 Patients with Hepatic Impairment
10 OVERDOSAGE
10.1 Manifestations
10.2 Management
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.2 Relapsed or Refractory APL
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DIFFERENTIATION SYNDROME AND CARDIAC CONDUCTION ABNORMALITIES
Differentiation Syndrome: Patients with acute promyelocytic leukemia (APL) treated with arsenic trioxide injection have experienced symptoms of differentiation syndrome, which can be fatal if not treated. Symptoms may include fever, dyspnea, acute respiratory distress, pulmonary infiltrates, pleural or pericardial effusions, weight gain or peripheral edema, hypotension, and renal, hepatic, or multi-organ dysfunction, in the presence or absence of leukocytosis. If differentiation syndrome is suspected, immediately initiate high-dose corticosteroid therapy and hemodynamic monitoring until resolution of signs and symptoms. Temporary discontinuation of arsenic trioxide injection may be required [see Warnings and Precautions ( 5.1) and Adverse Reactions ( 6.1) ].
Cardiac Conduction Abnormalities: Arsenic trioxide can cause QTc interval prolongation, complete atrioventricular block, and a torsade de pointes-type ventricular arrhythmia, which can be fatal. Before initiating therapy, assess the QTc interval, correct pre-existing electrolyte abnormalities, and consider discontinuing drugs known to prolong QTc interval. Do not administer arsenic trioxide injection to patients with ventricular arrhythmia or prolonged QTcF [see Warnings and Precautions ( 5.2) ].
-
1 INDICATIONS AND USAGE
1.2 Relapsed or Refractory APL
Arsenic trioxide injection is indicated for induction of remission and consolidation in patients with APL who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Relapsed or Refractory APL
A treatment course including arsenic trioxide injection monotherapy for patients with relapsed or refractory APL consists of 1 induction cycle and 1 consolidation cycle [see Clinical Studies ( 14.2) ].
- For the induction cycle, the recommended dose of arsenic trioxide injection is 0.15 mg/kg intravenously daily until bone marrow remission or up to a maximum of 60 days.
- For the consolidation cycle, the recommended dose of arsenic trioxide injection is 0.15 mg/kg intravenously daily for 25 doses over a period of up to 5 weeks. Begin consolidation 3 to 6 weeks after completion of induction therapy.
2.2 Dose Modifications for Toxicities
During induction therapy, monitor coagulation studies, blood counts, and chemistries at least 2-3 times per week through recovery. During consolidation, monitor at least weekly. Management of some adverse reactions may require dose interruption, dose reduction, or permanent discontinuation of arsenic trioxide injection [see Warnings and Precautions ( 5) and Adverse Reactions ( 6) ]. Table 2 shows the dose modifications for toxicity due to arsenic trioxide injection when used alone.
Table 2: Dose Adjustments for Adverse Reactions Adverse Reaction(s) Dose Modification Differentiation syndrome, defined by the presence of 2 or more of the following:
- Unexplained fever
- Dyspnea
- Pleural and/or pericardial effusion
- Pulmonary infiltrates
- Renal failure
- Hypotension
- Weight gain greater than 5 kg- Temporarily withhold arsenic trioxide injection. Treat with dexamethasone 10 mg intravenously every 12 hours until the resolution of signs and symptoms for a minimum of 3 days.
- Resume treatment when the clinical condition improves and reduce the dose of the withheld drug(s) by 50%.
- Increase the dose of the withheld drug(s) to the recommended dosage after 7 days in the absence of recurrence of symptoms of differentiation syndrome.
- If symptoms re-appear, decrease arsenic trioxide injection to the previous dose.
QTc Prolongation greater than 450 msec for men or greater than 460 msec for women: - Withhold treatment with arsenic trioxide injection and any medication known to prolong the QTc interval.
- Replete electrolytes.
- After the QTc normalizes, resume treatment with arsenic trioxide injection at a 50% reduced dose (0.075 mg/kg once daily) for 7 days.
- If the 50% reduced dose is tolerated for 7 days (in the absence of QTc prolongation), increase the dose of arsenic trioxide injection to 0.11 mg/kg once daily for 7 days.
- The dose of arsenic trioxide injection can be increased to 0.15 mg/kg in the absence of QTc prolongation during that 14-day dose-escalation period.
Hepatotoxicity, defined by 1 or more of the following:
- Total bilirubin (TB) greater than 3 times the upper limit of normal (ULN)
- Aspartate aminotransferase (AST) greater than 5 times the ULN
- Alkaline phosphatase (AP) greater than 5 times the ULN- Withhold treatment with arsenic trioxide injection.
- Resume treatment at a 50% reduced dose of the withheld drug(s) when TB is less than 1.5 times the ULN and AP/AST are less than 3 times the ULN.
- Increase the dose of the withheld drug back to the recommended dosage after 7 days on the reduced dose in the absence of worsening of hepatotoxicity.
- Discontinue the withheld drug permanently if hepatotoxicity recurs.
Other severe or life- threatening (grade 3-4) nonhematologic reactions - Temporarily withhold arsenic trioxide injection.
- When the adverse reaction resolves to no more than mild (grade 1), resume arsenic trioxide injection reduced by 2 dose levels (see Table 3 below).
Moderate (grade 2) nonhematologic reactions - Reduce the dose of arsenic trioxide injection by 1 dose level (see Table 3 below).
Leukocytosis (WBC count greater than 10 Gi/L) - Administer hydroxyurea.
- Hydroxyurea may be discontinued when the WBC declines below 10 Gi/L.
Myelosuppression, defined by 1 or more of the following:
- absolute neutrophil count less than 1 Gi/L
- platelets less than 50 Gi/L lasting more than 5 weeks- Consider reducing the dose of arsenic trioxide injection by 1 dose level (see Table 3 below).
- If myelosuppression lasts 50 days or occurs on 2 consecutive cycles, assess a marrow aspirate for remission status. In the case of molecular remission, resume arsenic trioxide injection at 1 dose level lower (see Table 3 below).
Table 3: Dose Reduction Levels for Hematologic and Nonhematologic Toxicities Dose Level Arsenic trioxide injection
mg/kg intravenously once dailyStarting level 0.15 -1 0.11 -2 0.10 -3 0.075 2.3 Instructions for Preparation and Intravenous Administration
Reconstitution
Dilute arsenic trioxide injection with 100 to 250 mL 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP, using proper aseptic technique, immediately after withdrawal from the vial. Do not save any unused portions for later administration.
After dilution, arsenic trioxide injection is chemically and physically stable when stored for 24 hours at room temperature and 48 hours when refrigerated.
Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Administer arsenic trioxide injection intravenously over 2 hours. The infusion duration may be extended up to 4 hours if acute vasomotor reactions are observed. A central venous catheter is not required.
The arsenic trioxide injection vial is single-dose and does not contain any preservatives. Unused portions of each vial should be discarded properly. Do not mix arsenic trioxide injection with other medications.
Safe Handling Procedures
Arsenic trioxide injection is a cytotoxic drug. Follow applicable special handling and disposal procedures. 1
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Differentiation Syndrome
Differentiation syndrome, which may be life-threatening or fatal, has been observed in patients with acute promyelocytic leukemia (APL) treated with arsenic trioxide injection. In clinical trials, 16-23% of patients treated with arsenic trioxide injection for APL developed differentiation syndrome. Symptoms include unexplained fever, dyspnea, hypoxia, pulmonary infiltrates, pleural or pericardial effusion, weight gain, peripheral edema, hypotension, renal insufficiency, hepatopathy and multi-organ dysfunction. Differentiation syndrome has been observed with and without concomitant hyperleukocytosis, and it has occurred as early as day 1 of induction to as late as the second month induction therapy.
At the first signs of differentiation syndrome, interrupt treatment with arsenic trioxide injection and administer dexamethasone 10 mg intravenously twice daily. Continue high-dose steroids until signs and symptoms have abated for at least 3 days [see Dosage and Administration ( 2.2) ].
5.2 Cardiac Conduction Abnormalities
Patients treated with arsenic trioxide injection can develop QTc prolongation, torsade de pointes, and complete heart block. In the clinical trial of patients with relapsed or refractory APL treated with arsenic trioxide injection monotherapy, 40% had at least one ECG tracing with a QTc interval greater than 500 msec. A prolonged QTc was observed between 1 and 5 weeks after start of arsenic trioxide injection infusion, and it usually resolved by 8 weeks after arsenic trioxide injection infusion. There are no data on the effect of arsenic trioxide injection on the QTc interval during the infusion of the drug.
The risk of torsade de pointes is related to the extent of QTc prolongation, concomitant administration of QTc prolonging drugs, a history of torsade de pointes, pre-existing QTc interval prolongation, congestive heart failure, administration of potassium-wasting diuretics, or other conditions that result in hypokalemia or hypomagnesemia. The risk may be increased when arsenic trioxide injection is coadministered with medications that can lead to electrolyte abnormalities (such as diuretics or amphotericin B) [see Drug Interactions ( 7) ].
Prior to initiating therapy with arsenic trioxide injection, assess the QTc interval by electrocardiogram, correct pre- existing electrolyte abnormalities, and consider discontinuing drugs known to prolong QTc interval. Do not administer arsenic trioxide injection to patients with ventricular arrhythmia or prolonged QTc. If possible, discontinue drugs that are known to prolong the QTc interval. If it is not possible to discontinue the interacting drug, perform cardiac monitoring frequently [see Drug Interactions ( 7) ]. During arsenic trioxide injection therapy, maintain potassium concentrations above 4 mEq/L and magnesium concentrations above 1.8 mg/dL. Monitor ECG weekly, and more frequently for clinically unstable patients.
For patients who develop a QTc greater than 500 msec, immediately withhold treatment with arsenic trioxide injection and any medication known to prolong the QTc interval. Correct electrolyte abnormalities. When the QTc normalizes, resume arsenic trioxide injection at a reduced dose [see Dosage and Administration ( 2.2) ].
5.3 Hepatotoxicity
During treatment with arsenic trioxide injection, monitor liver chemistries at least 2-3 times per week through recovery from toxicities. Withhold treatment with arsenic trioxide injection if elevations in AST), alkaline phosphatase, and/or serum bilirubin occur to greater than 5 times the upper limit of normal [see Dosage and Administration ( 2.2) ].
Long-term liver abnormalities can occur in APL patients treated with arsenic trioxide injection.
5.4 Carcinogenesis
The active ingredient of arsenic trioxide injection, arsenic trioxide, is a human carcinogen. Monitor patients for the development of second primary malignancies.
5.5 Embryo-Fetal Toxicity
Arsenic trioxide injection can cause fetal harm when administered to a pregnant woman. Arsenic trioxide was embryolethal and teratogenic in rats when administered on gestation day 9 at a dose approximately 10 times the recommended human daily dose on a mg/m2 basis. A related trivalent arsenic, sodium arsenite, produced teratogenicity when administered during gestation in mice at a dose approximately 5 times the projected human dose on a mg/m2 basis and in hamsters at an intravenous dose approximately equivalent to the projected human daily dose on a mg/m2 basis. Advise pregnant women of the potential risk to a fetus. Advise females and males of reproductive potential to use effective contraception during and after treatment with arsenic trioxide injection [see Use in Specific Populations ( 8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling.
- Differentiation Syndrome [see Warnings and Precautions ( 5.1) ]
- Cardiac Conduction Abnormalities [see Warnings and Precautions ( 5.2) ]
- Hepatotoxicity [see Warnings and Precautions ( 5.3) ]
- Carcinogenesis [see Warnings and Precautions ( 5.4) ]
- Embryo-Fetal Toxicity [see Warnings and Precautions ( 5.5) ]
6.2 Postmarketing Experience6.2 Postmarketing Experience
The following reactions have been reported from clinical trials and/or worldwide postmarketing surveillance. Because they are reported from a population of unknown size, precise estimates of frequency cannot be made.
Cardiac disorders: Ventricular extrasystoles in association with QT prolongation, and ventricular tachycardia in association with QT prolongation. including torsade de pointes, atrioventricular block, and congestive heart failure
Nervous system disorders: Peripheral neuropathy, paresis, seizures, confusion
Hematologic disorders: Pancytopenia, bone marrow necrosis
Infections and infestations: Herpes zoster
Investigations: Gamma-glutamyltransferase increased
Musculoskeletal and connective tissue disorders: Bone pain, myalgia, rhabdomyolysis
Respiratory, thoracic, and mediastinal disorders: Differentiation syndrome, like retinoic acid syndrome, has been reported with the use of arsenic trioxide injection for the treatment of malignancies other than APL [see Boxed Warning].
Ear and labyrinth disorders: Deafness
Neoplasms benign, malignant and unspecified: Melanoma, pancreatic cancer, squamous cell carcinoma
Skin and subcutaneous tissue disorders: Toxic epidermal necrolysis
-
7 DRUG INTERACTIONS
Drugs That Can Prolong the QT/QTc Interval
Concomitant use of these drugs and arsenic trioxide injection may increase the risk of serious QT/QTc interval prolongation. Discontinue or replace with an alternative drug that does not prolong the QT/QTc interval while patient is using arsenic trioxide injection. Monitor ECGs more frequently in patients when it is not feasible to avoid concomitant use.
Drugs That Can Lead to Electrolyte Abnormalities
Electrolyte abnormalities increase the risk of serious QT/QTc interval prolongation. Avoid concomitant administration of drugs that can lead to electrolyte abnormalities. Monitor electrolytes more frequently in patients who must receive concomitant use of these drugs and arsenic trioxide injection.
Drugs That Can Lead to Hepatotoxicity
Concomitant use of these drugs and arsenic trioxide injection, particularly when given in combination with tretinoin, may increase the risk of serious hepatotoxicity. Discontinue or replace with an alternative drug that does not cause hepatotoxicity while the patient is using arsenic trioxide injection. Monitor liver function tests more frequently in patients when it is not feasible to avoid concomitant use.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on the mechanism of action [see Clinical Pharmacology ( 12.1) ] and findings in animal studies, arsenic trioxide injection can cause fetal harm when administered to a pregnant woman. Arsenic trioxide was embryolethal and teratogenic in rats when administered on gestation day 9 at a dose approximately 10 times the recommended human daily dose on a mg/m 2 basis ( see Data). A related trivalent arsenic, sodium arsenite, produced teratogenicity when administered during gestation in mice at a dose approximately 5 times the projected human dose on a mg/m 2 basis and in hamsters at an intravenous dose approximately equivalent to the projected human daily dose on a mg/m 2 basis. There are no studies with the use of arsenic trioxide injection in pregnant women, and limited published data on arsenic trioxide use during pregnancy are insufficient to inform a drug-associated risk of major birth defects and miscarriage. Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
One patient was reported to deliver a live infant with no reported congenital anomalies after receiving arsenic trioxide during the first five months of pregnancy. A second patient became pregnant three months after discontinuing arsenic trioxide and was reported to have a normal pregnancy outcome. A third patient was a pregnant healthcare provider who experienced dermal contact with liquid arsenic trioxide and had a normal pregnancy outcome after treatment and monitoring. A fourth patient who became pregnant while receiving arsenic trioxide had a miscarriage.
Animal Data
Studies in pregnant mice, rats, hamsters, and primates have shown that inorganic arsenicals cross the placental barrier when given orally or by injection. An increase in resorptions, neural-tube defects, anophthalmia and microphthalmia were observed in rats administered 10 mg/kg of arsenic trioxide on gestation day 9 (approximately 10 times the recommended human daily dose on a mg/m 2 basis). Similar findings occurred in mice administered a 10 mg/kg dose of a related trivalent arsenic, sodium arsenite (approximately 5 times the projected human dose on a mg/m 2 basis), on gestation days 6, 7, 8 or 9. Intravenous injection of 2 mg/kg sodium arsenite (approximately equivalent to the projected human daily dose on a mg/m2 basis) on gestation day 7 (the lowest dose tested) resulted in neural-tube defects in hamsters.
8.2 Lactation
Risk Summary
Arsenic trioxide is excreted in human milk. There is no information on the effects of arsenic trioxide on the breastfed child or on milk production. Because of the potential for serious adverse reactions in a breastfed child from arsenic trioxide injection, discontinue breastfeeding during treatment with arsenic trioxide injection and for two weeks after the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Arsenic trioxide injection can cause fetal harm when administered to a pregnant woman. Conduct pregnancy testing in females of reproductive potential prior to initiation of treatment with arsenic trioxide injection [see Use in Specific Populations ( 8.1) ].
Contraception
Females
Advise females of reproductive potential to use effective contraception during and after treatment with arsenic trioxide injection and for six months after the final dose.
Males
Advise males with female sexual partners of reproductive potential to use effective contraception during and after treatment with arsenic trioxide injection and for three months after the final dose.
Infertility
Males
Based on testicular toxicities including decreased testicular weight and impaired spermatogenesis observed in animal studies, arsenic trioxide injection may impair fertility in males of reproductive potential [see Nonclinical Toxicology ( 13.1) ].
8.4 Pediatric Use
The safety and efficacy of arsenic trioxide injection as a single agent for treatment of pediatric patients with relapsed or refractory APL is supported by the pivotal phase 2 study in 40 patients with relapsed or refractory APL. Five patients below the age of 18 years (age range: 5 to 16 years) were treated with arsenic trioxide injection at the recommended dose of 0.15 mg/kg/day. A literature review included an additional 17 patients treated with arsenic trioxide for relapsed or refractory APL, with ages ranging from 4 to 21 years. No differences in efficacy and safety were observed by age.
8.5 Geriatric Use
The safety and efficacy of arsenic trioxide injection as a single agent in older patients with relapsed or refractory APL is supported by the pivotal phase 2 study in 40 patients with relapsed or refractory APL. Six patients age 65 and above (age range: 65 to 73 years) were treated with arsenic trioxide injection at the recommended dose. A literature review included an additional 4 patients treated with arsenic trioxide for relapsed or refractory APL with ages ranging from 69 to 72 years. No differences in efficacy and safety were observed by age.
8.6 Patients with Renal Impairment
Exposure of arsenic trioxide may be higher in patients with severe renal impairment [see Clinical Pharmacology (12.3)] . Patients with severe renal impairment (creatinine clearance less than 30 mL/min) should be monitored for toxicity when these patients are treated with arsenic trioxide for injection, and a dose reduction may be warranted.
The use of arsenic trioxide for injection in patients on dialysis has not been studied.
8.7 Patients with Hepatic Impairment
Since limited data are available across all hepatic impairment groups, caution is advised in the use of arsenic trioxide for injection in patients with hepatic impairment [see Clinical Pharmacology(12.3)] . Monitor patients with severe hepatic impairment (Child-Pugh Class C) who are treated with arsenic trioxide for injection for toxicity.
-
10 OVERDOSAGE
10.1 Manifestations
Manifestations of Arsenic Trioxide for Injection overdosage include convulsions, muscle weakness and confusion.
10.2 Management
If symptoms of arsenic trioxide injection overdosage develop, the injection should be immediately discontinued and chelation therapy should be considered.
A conventional protocol for acute arsenic intoxication includes dimercaprol administered at a dose of 3 mg/kg intramuscularly every 4 hours until immediate life-threatening toxicity has subsided. Thereafter, penicillamine at a dose of 250 mg orally, up to a maximum frequency of four times per day (≤ 1 g per day), may be given.
-
11 DESCRIPTION
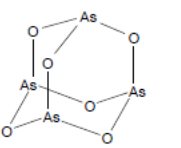
Arsenic Trioxide for Injection is a sterile injectable solution of arsenic trioxide. The molecular formula of the drug substance in the solid state is As 2O 3, with a molecular weight of 197.8 and has the following structural formula:
Arsenic Trioxide for Injection is available in 10 mL, single-use ampules containing 10 mg of arsenic trioxide. Arsenic Trioxide for Injection is formulated as a sterile, nonpyrogenic, clear solution of arsenic trioxide in water for injection using sodium hydroxide and dilute hydrochloric acid to adjust to pH 8. Arsenic Trioxide for Injection is preservative-free. Arsenic trioxide, the active ingredient, is present at a concentration of 1.0 mg/mL. Inactive ingredients and their respective approximate concentrations are sodium hydroxide (1.2 mg/mL) and hydrochloric acid, which is used to adjust the pH to 7.5 - 8.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of Arsenic Trioxide for Injection is not completely understood. Arsenic trioxide causes morphological changes and DNA fragmentation characteristic of apoptosis in NB4 human promyelocytic leukemia cells in vitro. Arsenic trioxide also causes damage or degradation of the fusion protein promyelocytic leukemia (PML)-retinoic acid receptor (RAR)-alpha.
12.2 Pharmacodynamics
Cardiac Electrophysiology
A dedicated QTc study was not performed with arsenic trioxide injection. However, in a single arm trial of arsenic trioxide injection (0.15 mg/kg daily), 16 of 40 patients (40%) had a QTc interval greater than 500 msec. Prolongation of the QTc was observed between 1 and 5 weeks after arsenic trioxide injection infusion, and then returned towards baseline by the end of 8 weeks after arsenic trioxide injection infusion.
12.3 Pharmacokinetics
The inorganic, lyophilized form of arsenic trioxide, when placed into solution, immediately forms the hydrolysis product arsenious acid (As III). As III is the pharmacologically active species of arsenic trioxide. Monomethylarsonic acid (MMA V), and dimethylarsinic acid (DMA V) are the main pentavalent metabolites formed during metabolism, in addition to arsenic acid (As V) a product of AsIIIoxidation. The pharmacokinetics of arsenical species ([As III], [As V], [MMA V], [DMA V]) were determined in 6 APL patients following once-daily doses of 0.15 mg/kg for 5 days per week. Over the total single-dose range of 7 to 32 mg (administered as 0.15 mg/kg), systemic exposure (AUC) appears to be linear. Peak plasma concentrations of arsenious acid (As III), the primary active arsenical species were reached at the end of infusion (2 hours). Plasma concentration of As III declined in a biphasic manner with a mean elimination half-life of 10 to 14 hours and is characterized by an initial rapid distribution phase followed by a slower terminal elimination phase. The daily exposure to As III(mean AUC0-24) was 194 ng·hr/mL (n=5) on Day 1 of Cycle 1 and 332 ng·hr/mL (n=6) on Day 25 of Cycle 1, which represents an approximate 2-fold accumulation. The primary pentavalent metabolites, MMA Vand DMA V, are slow to appear in plasma (approximately 10-24 hours after first administration of arsenic trioxide), but, due to their longer half-life, accumulate more upon multiple dosing than does As III. The mean estimated terminal elimination half-lives of the metabolites MMA V and DMA V are 32 hours and 72 hours, respectively. Approximate accumulation ranged from 1.4- to 8-fold following multiple dosing as compared to single dose administration. AsVis present in plasma only at relatively low levels.
Distribution
The volume of distribution (V ss) for As III is large (mean 562 L, N=10) indicating that As III is widely distributed throughout body tissues. V ss is also dependent on body weight and increases as body weight increases.
Elimination
Metabolism
Much of the As III is distributed to the tissues where it is methylated to the less cytotoxic metabolites, monomethylarsonic acid (MMA V) and dimethylarsinic acid (DMA V) by methyltransferases primarily in the liver. The metabolism of arsenic trioxide also involves oxidation of As III to As V, which may occur in numerous tissues via enzymatic or nonenzymatic processes. As V is present in plasma only at relatively low levels following administration of arsenic trioxide.
Excretion
Approximately 15% of the administered arsenic trioxide injection dose is excreted in the urine as unchanged As III. The methylated metabolites of As III (MMA V, DMA V) are primarily excreted in the urine. The total clearance of As III is 49 L/h and the renal clearance is 9 L/h. Clearance is not dependent on body weight or dose administered over the range of 7-32 mg.
Specific Populations
Patients with Renal Impairment
The effect of renal impairment on the pharmacokinetics of As III, As V, and the pentavalent metabolites MMA V and DMA V was evaluated in 20 patients with advanced malignancies. Patients were classified as having normal renal function (creatinine clearance [CrCl] > 80 mL/min, n=6), mild renal impairment (CrCl 50-80 mL/min, n=5), moderate renal impairment (CrCl 30-49 mL/min, n=6), or severe renal impairment (CrCl < 30 mL/min, n=3). Following twice weekly administration of 0.15 mg/kg over a 2-hour infusion, the mean AUC 0-∞ for As III was comparable among the normal, mild and moderate renal impairment groups. However, in the severe renal impairment group, the mean AUC 0-∞ for As III was approximately 48% higher than that in the normal group.
Systemic exposure to MMA V and DMA V tended to be larger in patients with renal impairment; however, the clinical consequences of this increased exposure are not known. As V plasma levels were generally below the limit of assay quantitation in patients with impaired renal function [see Use in Specific Populations ( 8.6) ]. The use of arsenic trioxide in patients on dialysis has not been studied.
Patients with Hepatic Impairment
The effect of pharmacokinetics of As III, As V, and the pentavalent metabolites MMA V and DMA V was evaluated following administration of 0.25-0.50 mg/kg of arsenic trioxide in patients with hepatocellular carcinoma. Patients were classified as having normal hepatic function (n=4), mild hepatic impairment (Child-Pugh class A, n=12), moderate hepatic impairment (Child-Pugh class B, n=3), or severe hepatic impairment (Child-Pugh class C, n=1). No clear trend toward an increase in systemic exposure to As III, As V, MMA V or DMA V was observed with decreasing level of hepatic function as assessed by dose-normalized (per mg dose) AUC in the mild and moderate hepatic impairment groups. However, the one patient with severe hepatic impairment had mean dose-normalized AUC 0-24 and C max values 40% and 70% higher, respectively, than those patients with normal hepatic function. The mean dose-normalized trough plasma levels for both MMAVand DMAV in this severely hepatically impaired patient were 2.2-fold and 4.7-fold higher, respectively, than those in the patients with normal hepatic function [see Use in Specific Populations ( 8.7) ].
Pediatric Patients
Following IV administration of 0.15 mg/kg/day of arsenic trioxide in 10 APL patients (median age = 13.5 years, range 4-20 years), the daily exposure to As III (mean AUC 0-24h) was 317 ng·hr/mL on Day 1 of Cycle 1 [see Use in Specific Populations ( 8.4) ].
Drug Interaction Studies
No formal assessments of pharmacokinetic drug-drug interactions between arsenic trioxide injection and other drugs have been conducted. The methyltransferases responsible for metabolizing arsenic trioxide are not members of the cytochrome P450 family of isoenzymes. In vitro incubation of arsenic trioxide with human liver microsomes showed no inhibitory activity on substrates of the major cytochrome P450 (CYP) enzymes such as 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4/5, and 4A9/11. The pharmacokinetics of drugs that are substrates for these CYP enzymes are not expected to be affected by concomitant treatment with arsenic trioxide.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with arsenic trioxide injection by intravenous administration [see Warnings and Precautions ( 5.4) ].
Arsenic trioxide and trivalent arsenite salts have not been demonstrated to be mutagenic to bacteria, yeast or mammalian cells. Arsenite salts are clastogenic in vitro (human fibroblast, human lymphocytes, Chinese hamster ovary cells, Chinese hamster V79 lung cells). Trivalent arsenic was genotoxic in the chromosome aberrations assay and micronucleus bone marrow assay in mice.
The effect of arsenic on fertility has not been adequately studied in humans. Decreased testicular weight and impaired spermatogenesis have been reported in animal studies. Male Wistar rat pups were administered 1.5 mg/kg sodium arsenite solution via the intraperitoneal route from postnatal days 1 to 14 and testes were collected for evaluation on postnatal days 15, 21, and 50. Results of this study revealed an altered morphology of the seminiferous tubules along with degeneration of spermatogenic cells, increased number of sperm with abnormal morphology, and decreased sperm counts. In beagle dogs administered intravenous arsenic trioxide for 90 days, reduced inner cell layers within seminiferous tubules and significantly decreased numbers of spermatocytes, spermatozoa, and sperm cells were observed at doses of 1 mg/kg/day and higher. The 1 mg/kg/day dose is approximately 3 times the recommended human daily dose on a mg/m basis.
-
14 CLINICAL STUDIES
14.2 Relapsed or Refractory APL
Arsenic trioxide injection has been investigated in Study PLRXAS01, an open-label, single-arm trial in 40 relapsed or refractory APL patients, previously treated with an anthracycline and a retinoid regimen. Patients received arsenic trioxide injection 0.15 mg/kg/day intravenously over 1 to 2 hours until the bone marrow was cleared of leukemic cells or up to a maximum of 60 days. The CR (absence of visible leukemic cells in bone marrow and peripheral recovery of platelets and white blood cells with a confirmatory bone marrow ≥ 30 days later) rate in this population of previously treated patients was 28 of 40 (70%). Among the 22 patients who had relapsed less than one year after treatment with tretinoin, there were 18 complete responders (82%). Of the 18 patients receiving arsenic trioxide injection ≥ one year from tretinoin treatment, there were 10 complete responders (55%). The median time to bone marrow remission was 44 days and to onset of CR was 53 days. Three of 5 children, 5 years or older, achieved CR. No children less than 5 years old were treated.
Three to six weeks following bone marrow remission, 31 patients received consolidation therapy with arsenic trioxide injection, at the same dose, for 25 additional days over a period up to 5 weeks. In follow-up treatment, 18 patients received further arsenic trioxide injection as a maintenance course. Fifteen patients had bone marrow transplants. At last follow-up, 27 of 40 patients were alive with a median follow-up time of 484 days (range 280 to 755) and 23 of 40 patients remained in complete response with a median follow-up time of 483 days (range 280 to 755).
Cytogenetic conversion to no detection of the APL chromosome rearrangement was observed in 24 of 28 (86%) patients who met the response criteria defined above, in 5 of 5 (100%) patients who met some, but not all, of the response criteria, and 3 of 7 (43%) of patients who did not respond. RT-PCR conversions to no detection of the APL gene rearrangement were demonstrated in 22 of 28 (79%) of patients who met the response criteria, in 3 of 5 (60%) of patients who met some, but not all, of the response criteria, and in 2 of 7 (29%) of patients who did not respond.
Responses were seen across all age groups tested, ranging from 6 to 72 years. The ability to achieve a CR was similar for both genders. There were insufficient patients of Black, Hispanic, or Asian derivation to estimate relative response rates in these groups, but responses were seen in members of each group.
-
15 REFERENCES
- “Hazardous Drugs”, OSHA. [Accessed on February 12, 2015 from http://www.osha.gov/SLTC/hazardousdrugs/index.html]
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
- Differentiation Syndrome
Advise patients that symptoms of APL differentiation syndrome include fever, sudden weight gain, dizziness/lightheadedness, labored breathing, and accumulation of fluid in the lungs, heart, and chest. This syndrome is managed by immediate treatment with high dose corticosteroids. Advise patients to immediately report any of these symptoms.
- ECG Abnormalities – QT Prolongation
Advise patients that arsenic trioxide injection may cause ECG abnormalities, including QT prolongation. QT prolongation is an increase in the time it takes the heart to relax between beats. If extreme, this prolongation has the potential to cause fainting, irregular heartbeat, or more serious side effects. Advise patients to immediately report any of these symptoms. Advise patients to provide a complete list of current medications as caution should be taken when arsenic trioxide injection is coadministered with other medications that can cause QT prolongation or lead to electrolyte abnormalities.
- Other Side Effects
Advise patients of the expected adverse reactions of arsenic trioxide injection. Most patients in clinical trials experienced some drug-related toxicity, most commonly leukocytosis, gastrointestinal symptoms (nausea, vomiting, diarrhea, and abdominal pain), fatigue, edema, hyperglycemia, dyspnea, cough, rash or itching, headaches, and dizziness. These adverse reactions have not been observed to be permanent or irreversible, nor do they usually require interruption of therapy. Advise patients to call their physician at the onset of any treatment related adverse reactions.
- Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider with a known or suspected pregnancy [see Warnings and Precautions 5.5 and Use in Specific Populations 8.1) ].
Advise females and males of reproductive potential to use effective contraception during and after treatment with arsenic trioxide injection. Advise females to use effective contraception for six months and males to use effective contraception for three months after completing treatment with arsenic trioxide injection [see Use in Specific Populations ( 8.3) ].
- Potential Effect on Male Fertility
Advise male patients of the potential risk to future fertility following treatment with arsenic trioxide injection, as decreased testicular weight and impaired spermatogenesis have been reported in animal studies
- Lactation
Advise females to discontinue breastfeeding during treatment with arsenic trioxide injection and for two weeks after treatment with arsenic trioxide injection [see Use in Specific Populations ( 8.2) ].
Rx onlyManufactured for STI Pharma LLC
Newtown, PA 18940
Made in USA
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
ARSENIC TRIOXIDE
arsenic trioxide injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54879-027 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54879-027-11 10 in 1 CARTON 05/29/2019 1 NDC:54879-027-10 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209873 05/29/2019 Labeler - STI Pharma LLC (832714070) Registrant - STI Pharma LLC (832714070)


