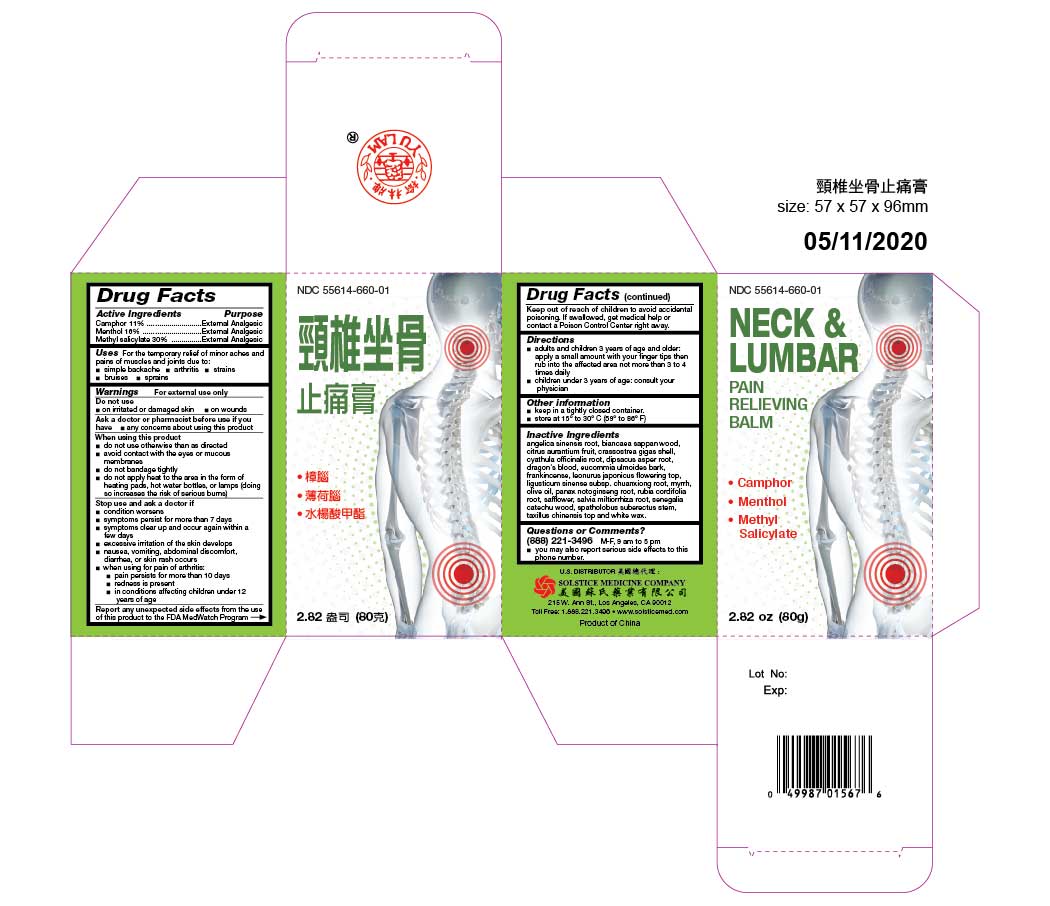
Label: NECK AND LUMBAR PAIN RELIEVING BALM- camphor, menthol, and methyl salicylate oil
-
Contains inactivated NDC Code(s)
NDC Code(s): 55614-660-01 - Packager: MADISON ONE ACME INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 18, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
■ condition worsens
■ symptoms persist for more than 7 days
■ symptoms clear up and occur again within a few days
■ excessive irritation of the skin develops
■ nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
■ you feel actual pain or experience blistering or burning after application (it is normal to feel a warming or cooling sensation)
■ when using for pain of arthritis:
■ pain persists for more than 10 days
■ redness is present
■ in conditions affecting children under 12 years of age - ADVERSE REACTIONS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
angelica sinensis root, biancaea sappan wood, citrus aurantium fruit, crassostrea gigas shell, cyathula officinalis root, dipsacus asper root, dragon's blood, eucommia ulmoides bark, frankincense, leonurus japonicus flowering top, ligusticum sinense subsp. chuanxiong root, myrrh, olive oil, panax notoginseng root, rubia cordifolia root, safflower, salvia miltiorrhiza root, senegalia catechu wood, spatholobus suberectus stem, taxillus chinensis top and white wax. - QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NECK AND LUMBAR PAIN RELIEVING BALM
camphor, menthol, and methyl salicylate oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55614-660 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 11 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 16 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 30 g in 100 g Inactive Ingredients Ingredient Name Strength ANGELICA SINENSIS ROOT (UNII: B66F4574UG) BIANCAEA SAPPAN WOOD (UNII: 086263LQDD) CITRUS AURANTIUM FRUIT (UNII: DQD16J2B5O) CRASSOSTREA GIGAS SHELL (UNII: 5I0R0U8ZL0) CYATHULA OFFICINALIS ROOT (UNII: BRM37UP34O) DIPSACUS ASPER ROOT (UNII: LB1GQP4253) DRAGON'S BLOOD (UNII: M3YJ2C28IC) EUCOMMIA ULMOIDES BARK (UNII: L878N1L0AR) FRANKINCENSE (UNII: R9XLF1R1WM) LEONURUS JAPONICUS FLOWERING TOP (UNII: V315CCZ08Z) LIGUSTICUM SINENSE SUBSP. CHUANXIONG ROOT (UNII: RR83T99U97) MYRRH (UNII: JC71GJ1F3L) OLIVE OIL (UNII: 6UYK2W1W1E) PANAX NOTOGINSENG ROOT (UNII: GQX1C1175U) RUBIA CORDIFOLIA ROOT (UNII: 4V873H15CG) SAFFLOWER (UNII: 4VBL71TY4Y) SALVIA MILTIORRHIZA ROOT (UNII: 1693AM5SBN) SENEGALIA CATECHU WOOD (UNII: CHL342Y4LA) SPATHOLOBUS SUBERECTUS STEM (UNII: N51VZ363BA) TAXILLUS CHINENSIS TOP (UNII: QJ523LD71P) WHITE WAX (UNII: 7G1J5DA97F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55614-660-01 1 in 1 BOX 05/15/2020 1 80 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/15/2020 Labeler - MADISON ONE ACME INC (096196758) Establishment Name Address ID/FEI Business Operations Zhejiang Dingtai Pharmaceutical Co., Ltd 420598724 manufacture(55614-660)