Label: SAFE AND SILKY NATURAL HAND SANITIZER- safe and silky fragrance hs lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 78235-010-01, 78235-010-02, 78235-010-03, 78235-010-04, view more78235-010-05, 78235-010-06, 78235-010-07, 78235-010-08 - Packager: CK Polymers LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 22, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
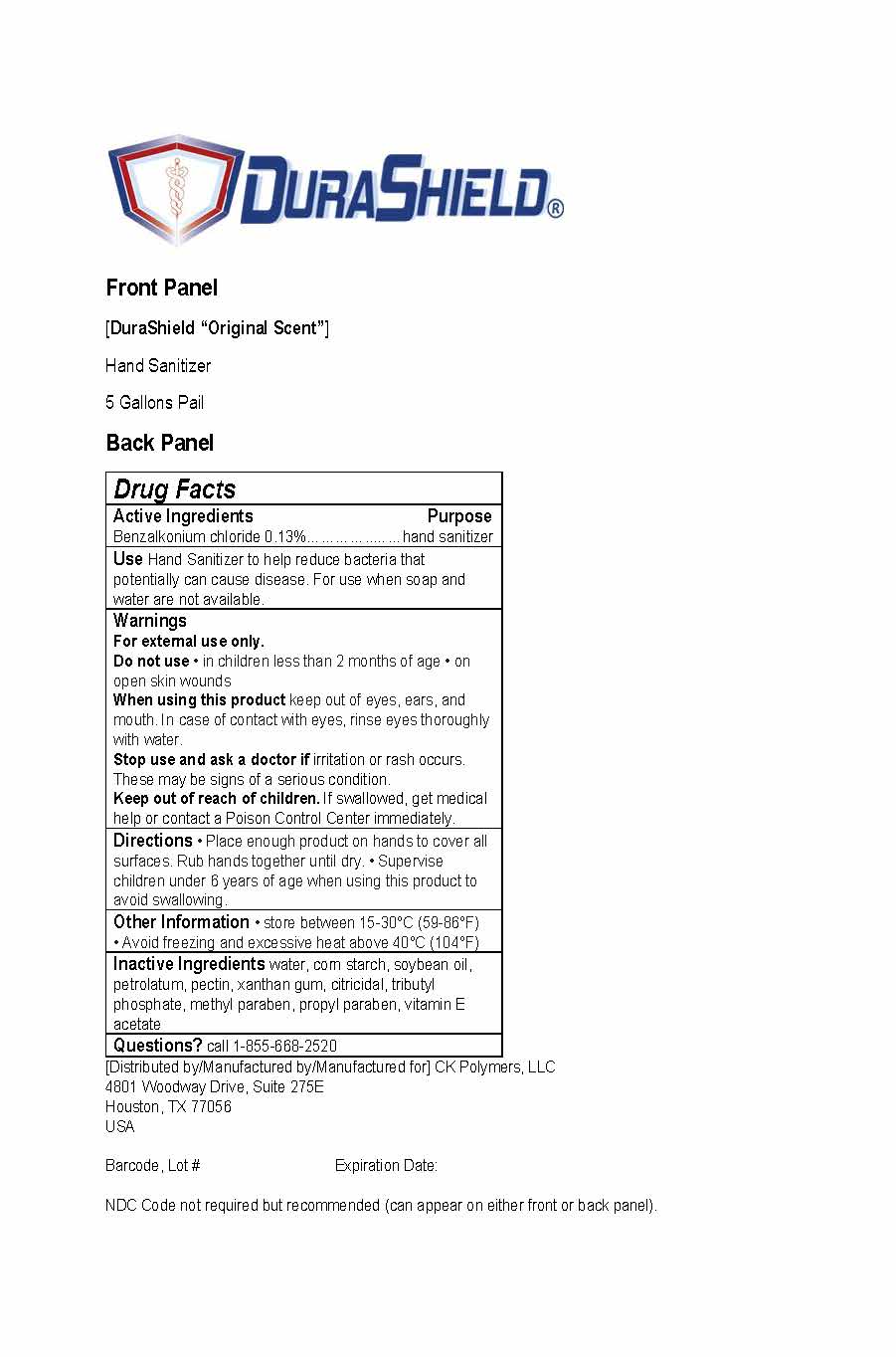
Safe & Silky Natural Hand Sanitizer
Use
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
Warnings
For external use only.
Do not use in children less than 2 months of age or on open skin wounds.
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Place enough product on hands to cover all surfaces. Rub hands together until dry. Supervise children under 6 years of age when using this product to avoid swallowing.
Other Information
Store between 15-30°C (59-86°F)
Avoid freezing and excessive heat above 40°C (104°F)
- 275 Gallon Tote - Safe & Silky Fragrance Hand Sanitizer - Label
- 55 Gallon Drum - Safe & Silky Natural Hand Sanitizer - Label
- 330 Gallon Tote - Safe & Silky Natural Hand Sanitizer - Label
- 1 Gallon Jug - Safe & Silky Natural Hand Sanitizer - Label
- 2OZ Bottlel - Safe & Silky Fragrance Hand Sanitizer - Label
- 4OZ Bottlel - Safe & Silky Fragrance Hand Sanitizer - Label
- 8OZ Bottlel - Safe & Silky Fragrance Hand Sanitizer - Label
- 5 Gallon Pail - Safe & Silky Fragrance Hand Sanitizer - Label
-
INGREDIENTS AND APPEARANCE
SAFE AND SILKY NATURAL HAND SANITIZER
safe and silky fragrance hs lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78235-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE .13 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CORN STARCH 3-E-DODECENYL SUCCINIC ANHYDRIDE MODIFIED (UNII: QG4MW19XYX) SOYBEAN OIL (UNII: 241ATL177A) PETROLATUM (UNII: 4T6H12BN9U) PECTIN (UNII: 89NA02M4RX) XANTHAN GUM (UNII: TTV12P4NEE) GRAPEFRUIT SEED OIL (UNII: 598D944HOL) TRIBUTYL PHOSPHITE (UNII: 498030B4AD) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78235-010-01 3785 mL in 1 JUG; Type 0: Not a Combination Product 10/06/2020 2 NDC:78235-010-02 208175 mL in 1 DRUM; Type 0: Not a Combination Product 10/06/2020 3 NDC:78235-010-03 18927.1 mL in 1 CONTAINER; Type 0: Not a Combination Product 10/06/2020 4 NDC:78235-010-04 1249050 mL in 1 CONTAINER; Type 0: Not a Combination Product 10/06/2020 5 NDC:78235-010-05 1040875 mL in 1 CONTAINER; Type 0: Not a Combination Product 10/09/2020 6 NDC:78235-010-06 60 mL in 1 CONTAINER; Type 0: Not a Combination Product 10/09/2020 7 NDC:78235-010-07 118 mL in 1 CONTAINER; Type 0: Not a Combination Product 10/09/2020 8 NDC:78235-010-08 237 mL in 1 CONTAINER; Type 0: Not a Combination Product 10/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 10/06/2020 Labeler - CK Polymers LLC (079790285) Establishment Name Address ID/FEI Business Operations CK Polymers LLC 079790285 manufacture(78235-010)