Label: NIGHT TIME COUGH AND COLD RELIEF- diphenhydramine hydrochloride, phenylephrine hydrochloride liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 68016-137-00 - Packager: Chain Drug Consortium, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 1, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Keep out of reach of children
- Uses
-
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on the skin
- to make a child sleepy
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for two weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or a pharmacist before taking this product.
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast feeding,
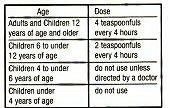
- Directions
- Other information
- Inactive ingredients
- Questions?
-
Product Label
NDC68016-137-00
*COMPARE TO THE ACTIVE INGREDIENTS IN DELSYM® NIGHT TIME COUGH and COLD
Premier Value®
NIGHT TIME
COUGH and COLD RELIEF
Diphenhydramine HCL .......................Antihistamine/Cough Suppressant
Phenylephrine HCL ...........................Nasal Decongestant
- Sneezing
- Runny Nose
- Cough
- Nasal Congestion
Grape Flavored Liquid
4 FL OZ (120 mL)INDEPENDENTLY TESTED SATISFACTION GUARANTEED PV
DO NOT USE IF PRINTED SEAL UNDER CAP IS TORN OR MISSING
*This product is not manufactured or distributed by Reckitt Benckiser Inc. distributor of Children’s Mucinex® Multisymptom Cold
If for Any reason you are not satisfied with this product, please return it to the store where purchased for a full refund.DISTRIBUTED BY
CHAIN DRUG CONSORTIUM
3301 NW BOCA RATON BLVD
SUITE 101, BOCA RATON, FL 33431
BX-009
-
INGREDIENTS AND APPEARANCE
NIGHT TIME COUGH AND COLD RELIEF
diphenhydramine hydrochloride, phenylephrine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-137 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 6.25 mg in 5 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) MALTITOL (UNII: D65DG142WK) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) Product Characteristics Color Score Shape Size Flavor GRAPE (Grape Flavored Liquid) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-137-00 120 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/01/2013 Labeler - Chain Drug Consortium, LLC (101668460) Registrant - AptaPharma Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(68016-137)