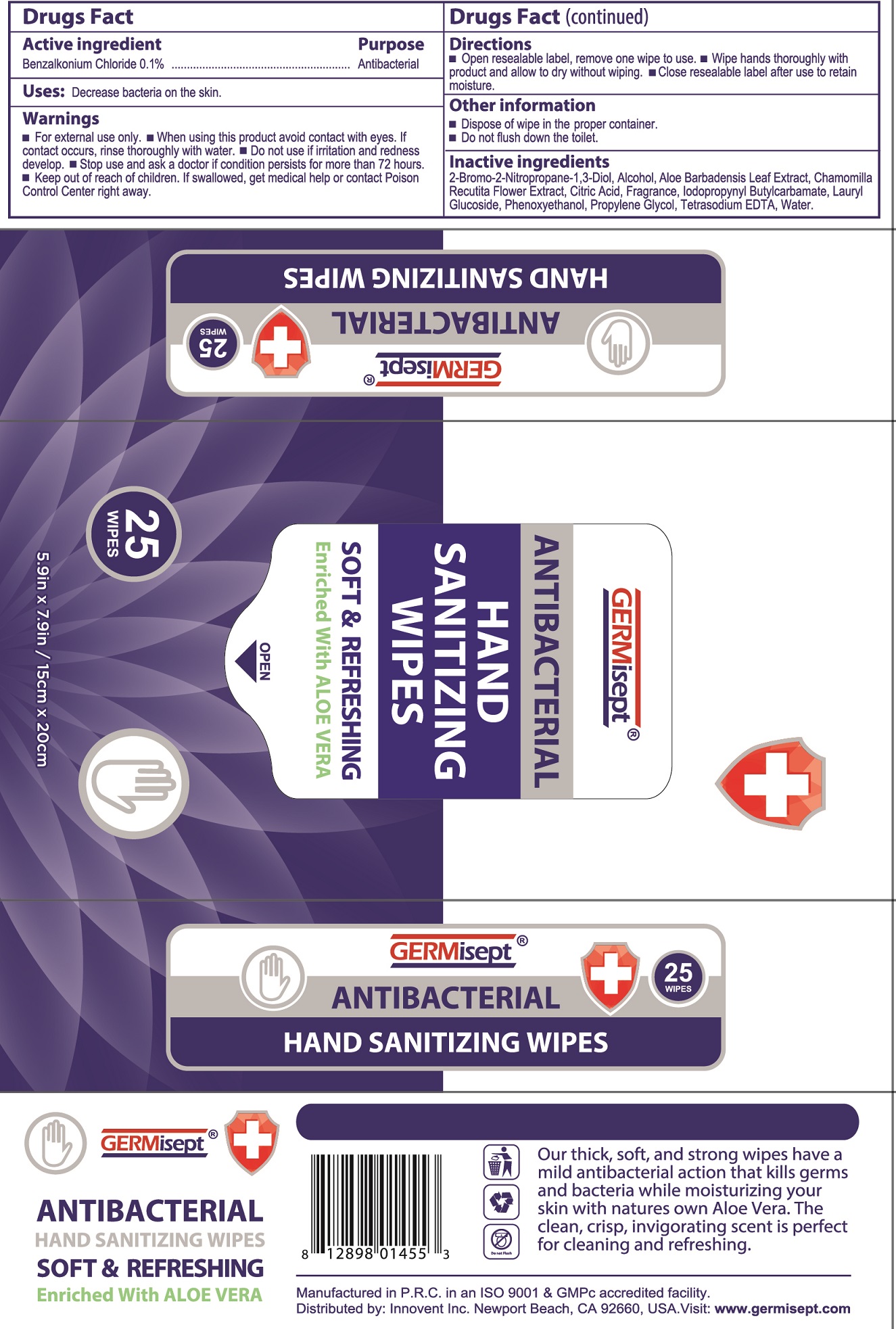
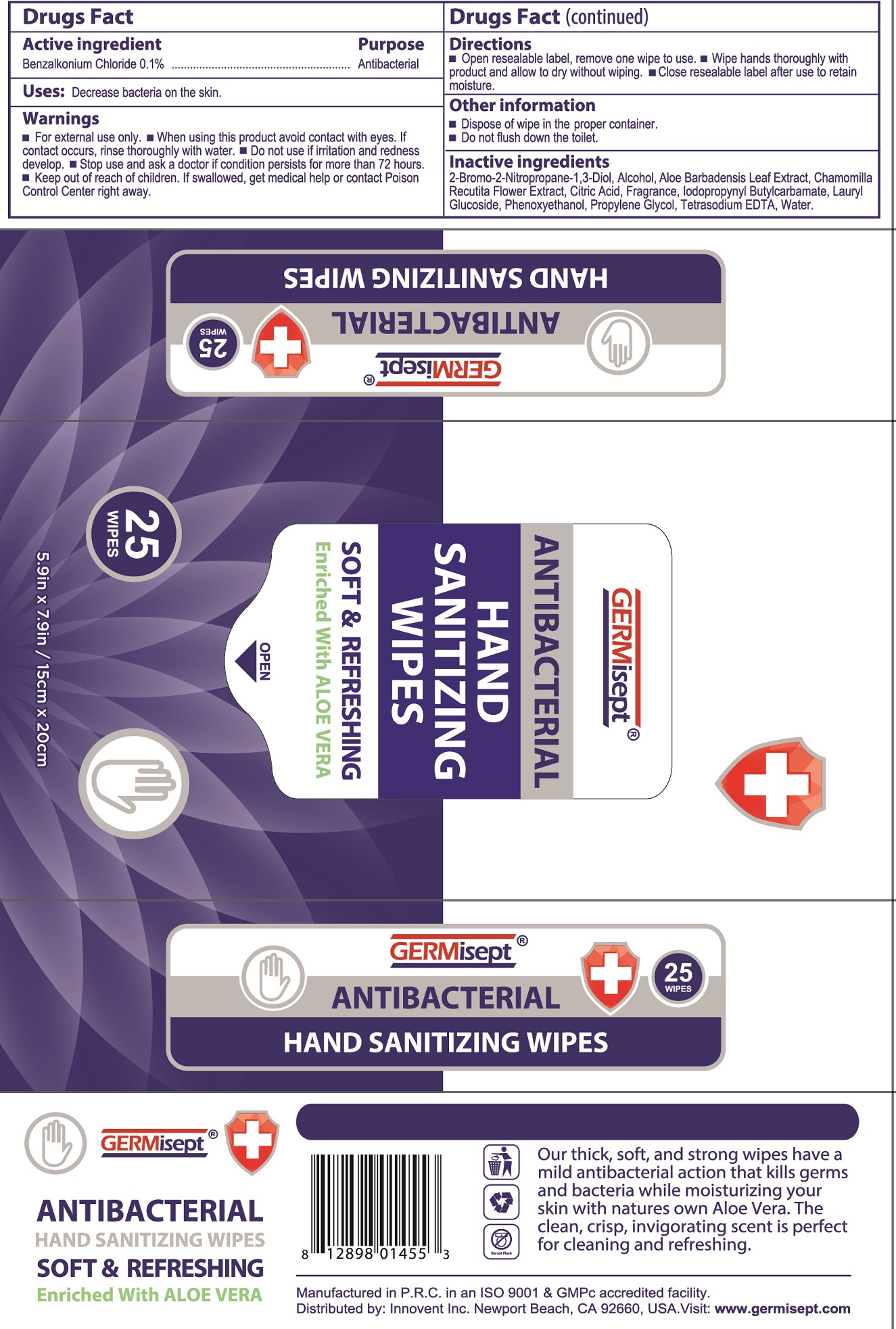
Label: GERMISEPT ANTIBACTERIAL HAND SANITIZING WIPES- benzalkonium chloride cloth
- NDC Code(s): 70335-304-00, 70335-304-01
- Packager: Innovent Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drugs Fact
- Active ingredient
- Uses:
- Warnings
- Directions
- Other information
- Inactive ingredients
- Package Labeling:
- Package Labeling: 50Ct
-
INGREDIENTS AND APPEARANCE
GERMISEPT ANTIBACTERIAL HAND SANITIZING WIPES
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70335-304 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength BRONOPOL (UNII: 6PU1E16C9W) ALCOHOL (UNII: 3K9958V90M) ALOE VERA LEAF (UNII: ZY81Z83H0X) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE SODIUM (UNII: MP1J8420LU) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70335-304-00 1 in 1 BOX 04/15/2020 11/30/2025 1 25 in 1 PACKAGE 1 4 mL in 1 PACKAGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:70335-304-01 1 in 1 BOX 06/15/2020 11/30/2025 2 50 in 1 PACKAGE 2 4 mL in 1 PACKAGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 04/15/2020 11/30/2025 Labeler - Innovent Inc (079973489)