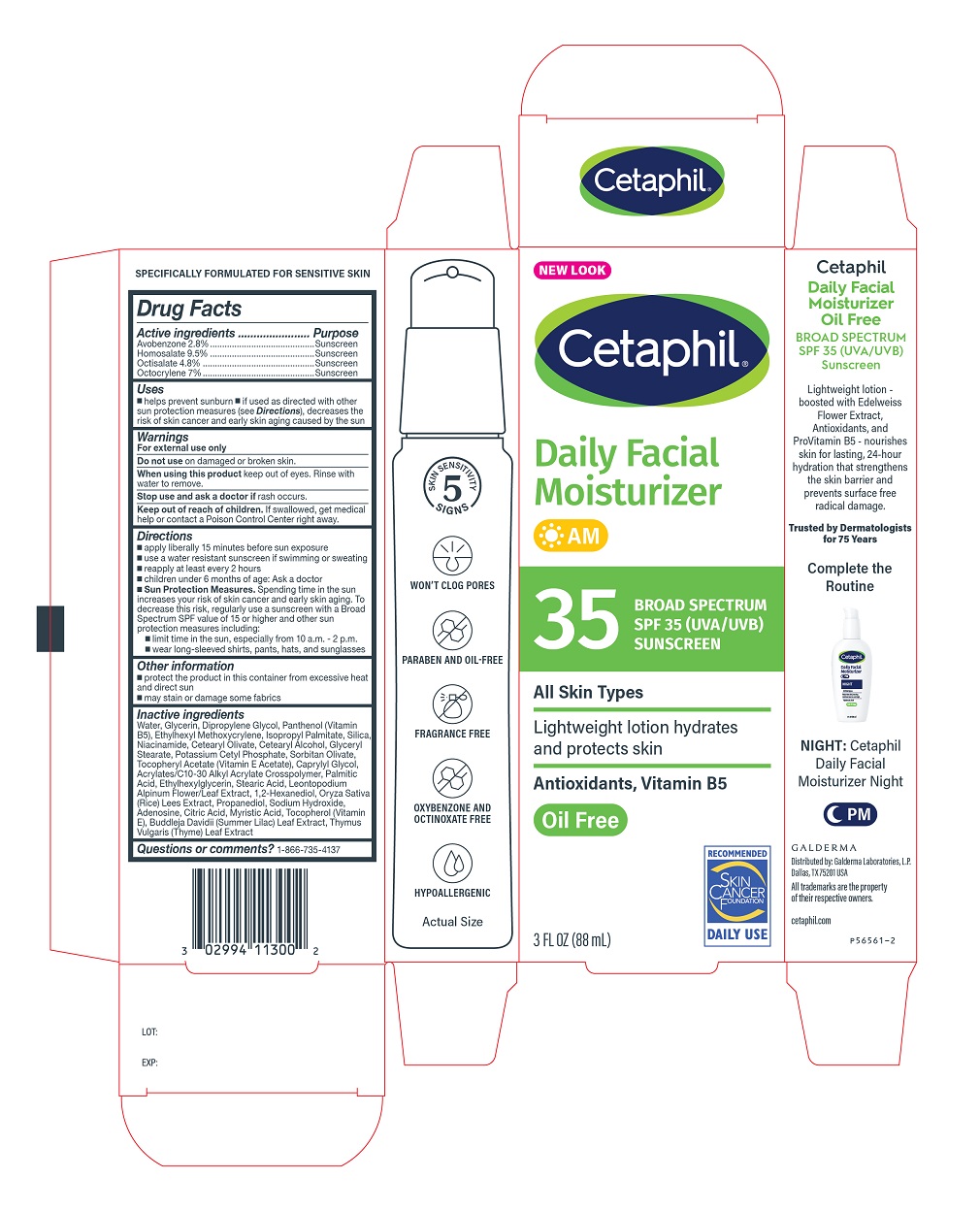
Label: CETAPHIL DAILY FACIAL MOISTURIZER WITH SUNCREEN SPF 35- avobenzone, homosalate, octisalate, octocrylene lotion
- NDC Code(s): 0299-4113-05
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- PURPOSE
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
-
Inactive Ingredients
Water, Glycerin, Dipropylene Glycol, Panthenol (Vitamin B5), Ethylhexyl Methoxycrylene, lsopropyl Palmitate, Silica, Niacinamide, Cetearyl 0livate, Cetearyl Alcohol, Glyceryl Stearate, Potassium Cetyl Phosphate, Sorbitan Olivate, Tocopheryl Acetate (Vitamin E Acetate), Caprylyl Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Palmitic Acid, Ethylhexylglycerin, Stearic Acid, Leontopodium Alpinum Flower/Leaf Extract, 1,2-Hexanediol, Oryza Saliva (Rice) Lees Extract, Propanediol, Sodium Hydroxide, Adenosine, Citric Acid, Myristic Acid, Tocopherol (Vitamin E), Buddleja Davidii (Summer Lilac) Leaf Extract, Thymus Vulgaris (Thyme) Leaf Extract
- Questions or comments?
-
Principal Display Panel - 3 FL OZ Carton
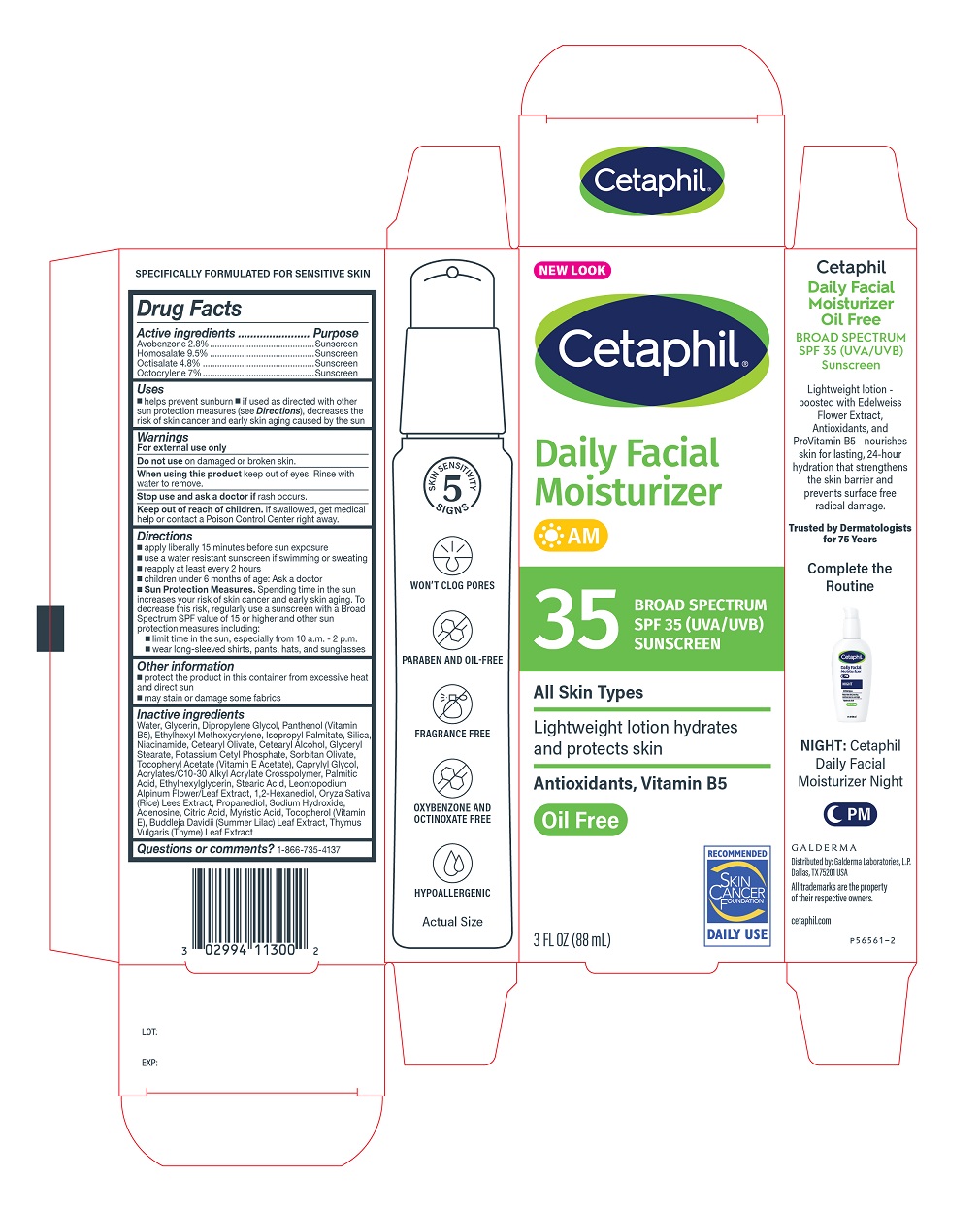
NEW LOOK
Cetaphil®
Daily Facial
Moisturizer
AM35 BROAD SPECTRUM
SPF 35 (UVA/UVB)
SUNSCREENAll Skin Types
Lightweight lotion
hydrates and protects skin
Antioxidants, Vitamin B5
Oil-FreeSkin Cancer Foundation logo
3 FL OZ (88 mL)
Distributed by:
Galderma Laboratories, L.P.
Dallas, Texas 75201 USA
All trademarks are the property of their respective owners.
cetaphil.com
P56561-2
-
INGREDIENTS AND APPEARANCE
CETAPHIL DAILY FACIAL MOISTURIZER WITH SUNCREEN SPF 35
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-4113 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 28 mg in 1 mL Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 95 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 48 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 70 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Dipropylene Glycol (UNII: E107L85C40) Panthenol (UNII: WV9CM0O67Z) Ethylhexyl Methoxycrylene (UNII: S3KFG6Q5X8) Isopropyl Palmitate (UNII: 8CRQ2TH63M) Silicon Dioxide (UNII: ETJ7Z6XBU4) Niacinamide (UNII: 25X51I8RD4) Cetearyl Olivate (UNII: 58B69Q84JO) Cetostearyl Alcohol (UNII: 2DMT128M1S) Glyceryl Monostearate (UNII: 230OU9XXE4) Potassium Cetyl Phosphate (UNII: 03KCY6P7UT) Sorbitan Olivate (UNII: MDL271E3GR) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Caprylyl Glycol (UNII: 00YIU5438U) Carbomer Interpolymer Type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Palmitic Acid (UNII: 2V16EO95H1) Ethylhexylglycerin (UNII: 147D247K3P) Stearic Acid (UNII: 4ELV7Z65AP) Leontopodium Nivale Subsp. Alpinum Flowering Top (UNII: QQC1AK06RK) 1,2-Hexanediol (UNII: TR046Y3K1G) Rice Germ (UNII: 7N2B70SFEZ) Propanediol (UNII: 5965N8W85T) Sodium Hydroxide (UNII: 55X04QC32I) Adenosine (UNII: K72T3FS567) Citric Acid Monohydrate (UNII: 2968PHW8QP) Myristic Acid (UNII: 0I3V7S25AW) Tocopherol (UNII: R0ZB2556P8) Buddleja Davidii Leaf (UNII: X380815D32) Thymus Vulgaris Leaf (UNII: GRX3499643) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-4113-05 1 in 1 CARTON 11/01/2020 1 88 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2020 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations ENGLEWOOD LAB, INC. 172198223 label(0299-4113) , manufacture(0299-4113) , pack(0299-4113)