Label: CYNERGY BARRIER- heptanoic acid solution
- NDC Code(s): 47593-445-16, 47593-445-17, 47593-445-21, 47593-445-88
- Packager: Ecolab Inc
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 21, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SAFE HANDLING WARNING
-
VETERINARY INDICATIONS
DIRECTIONS:
IMPORTANT: Do not further dilute with water or mix with any other teat dips. If product in dip cup becomes visibly dirty, discard contents and replenish with fresh product. Do not reuse or return any unused product to the original container.
Udder Prep: When using an udder wash step before milking, make sure to wash teats with appropriate udder wash solution using proper cleaning procedures. Teats should then be dried with single-service towels.
Directions for Teat Dipping
Pre-Milk Dipping: Before each cow is milked, and using fresh Cynergy Barrier, dip each teat full-length into the teat dip cup. Wipe teats after dipping using single-service towels to avoid contamination of milk.
Post-Milk Dipping: Using fresh Cynergy Barrier, dip each teat full-length into the teat dip cup. Do not wipe. Allow to air dry. Do not turn cows out in freezing weather until the product is completely dry. Expanded Usage: When freshening cows, begin dipping teats twice daily for about 10 days before calving.
PRECAUTION: Cynergy Barrier is not intended to cure or help the healing of chapped or irritated teats. As with any germicide, irritation or sensitization may occur in sensitive animals. In case of teat irritation or chapping, have the condition examined and, if necessary, treated by a veterinarian.
Consult your Ecolab representative for specific use instructions and recommended dispensing equipment.
-
OTHER SAFETY INFORMATION
For External Use Only
Wash hands thoroughly after handling.
Get medical advice/ attention if you feel unwell.
READ SAFETY DATA SHEET (SDS) BEFORE USING THIS PRODUCT
EMERGENCY HEALTH INFORMATION: 1 800 328 0026. If located outside the United States and
Canada, call collect 1 651 222 5352 (number is in the US).
-
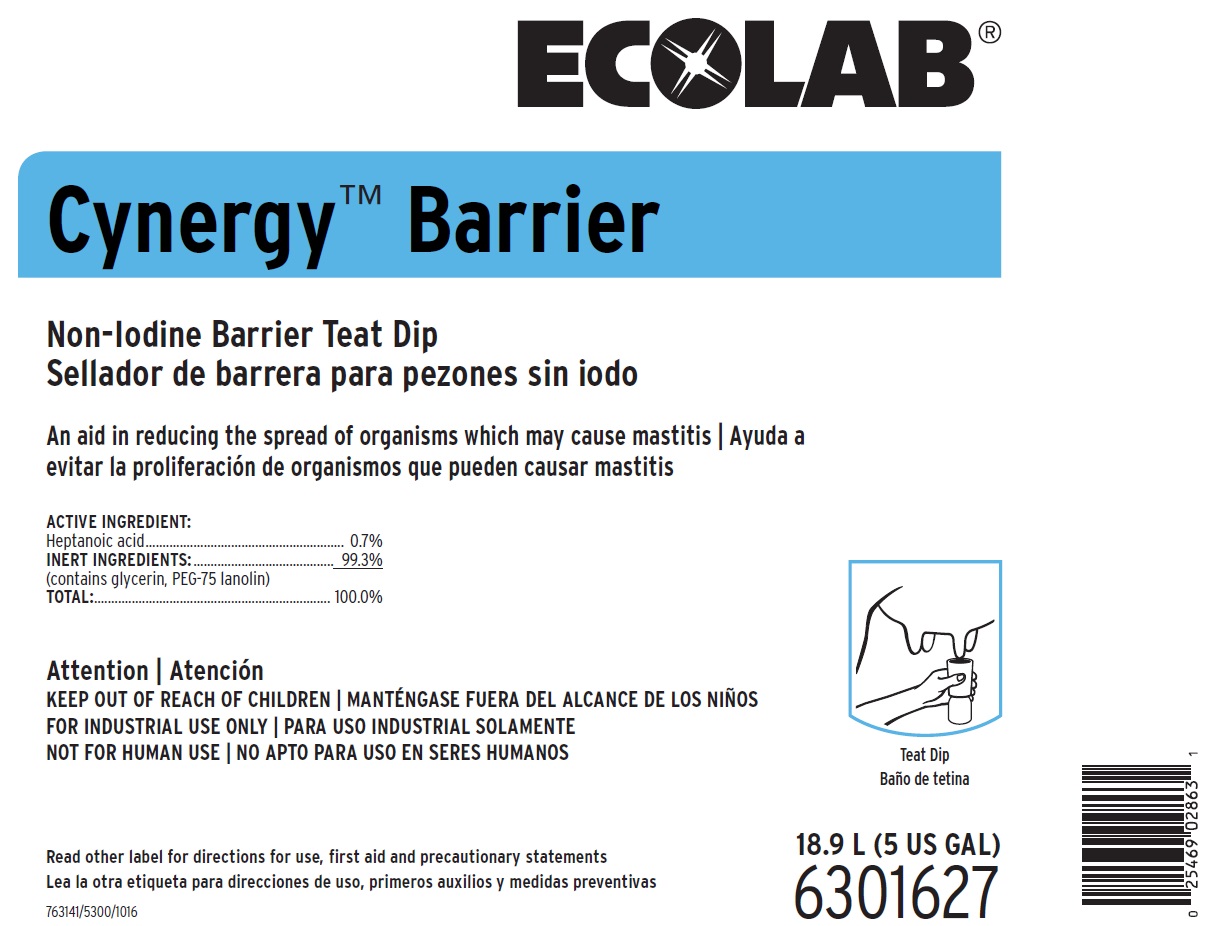
PRINCIPAL DISPLAY PANEL
ECOLAB®
CYNERGY™ BARRIER
Non-Iodine Barrier Teat Dip
An Aid in Reducing the Spread of Organisms Which May Cause Mastitis
ACTIVE INGREDIENT:
Heptanoic acid...................................................0.7%
INERT INGREDIENTS: ........................................99.3%
(contains glycerin, PEG-75 lanolin)
TOTAL:.............................................................100.0%
18.9 L (5 US GAL)

-
INGREDIENTS AND APPEARANCE
CYNERGY BARRIER
heptanoic acid solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:47593-445 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPTANOIC ACID (UNII: THE3YNP39D) (HEPTANOIC ACID - UNII:THE3YNP39D) HEPTANOIC ACID 7 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PEG-75 LANOLIN (UNII: 09179OX7TB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-445-16 18900 mL in 1 PAIL 2 NDC:47593-445-17 56800 mL in 1 DRUM 3 NDC:47593-445-21 208200 mL in 1 DRUM 4 NDC:47593-445-88 1041000 mL in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/23/2007 Labeler - Ecolab Inc (006154611)


