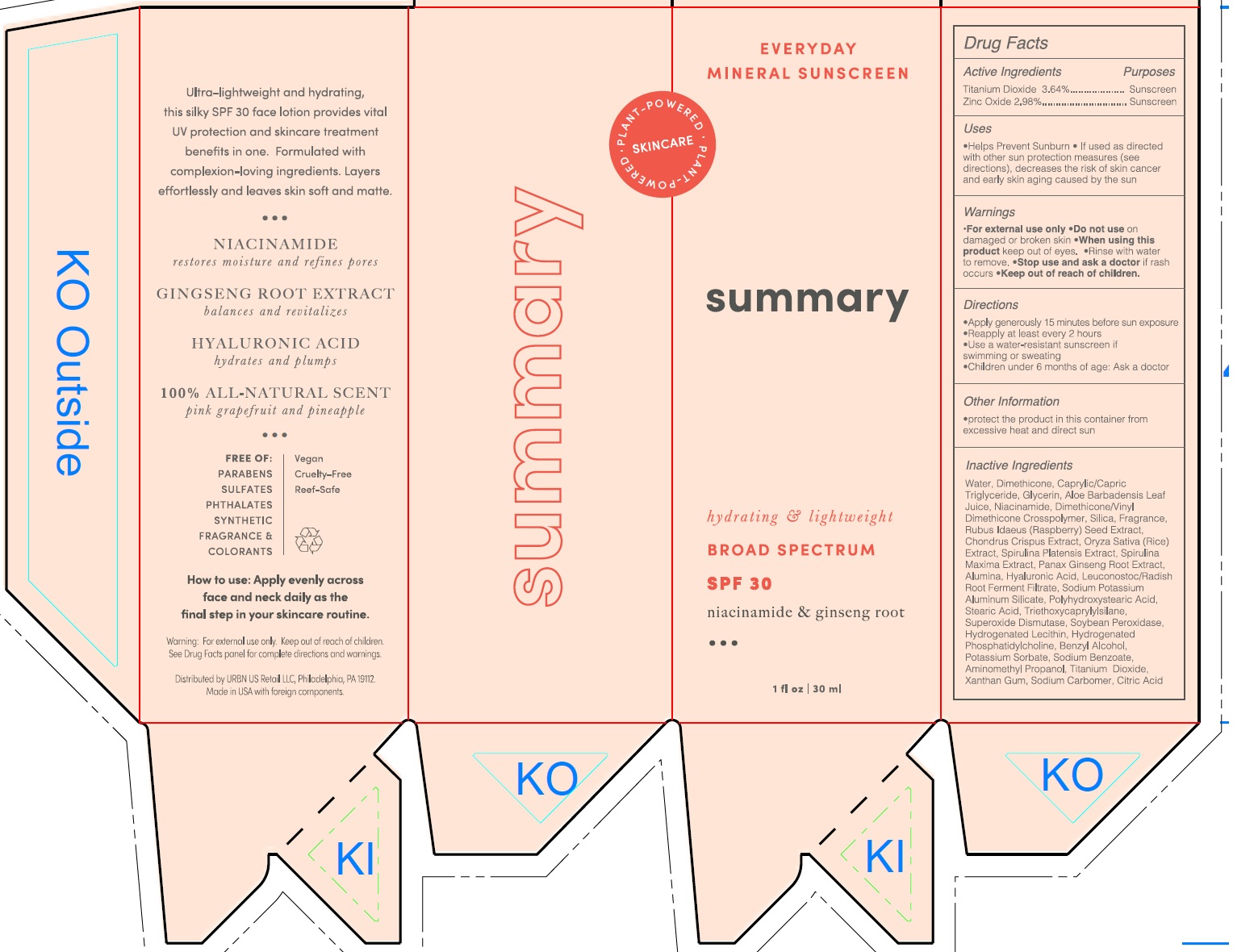
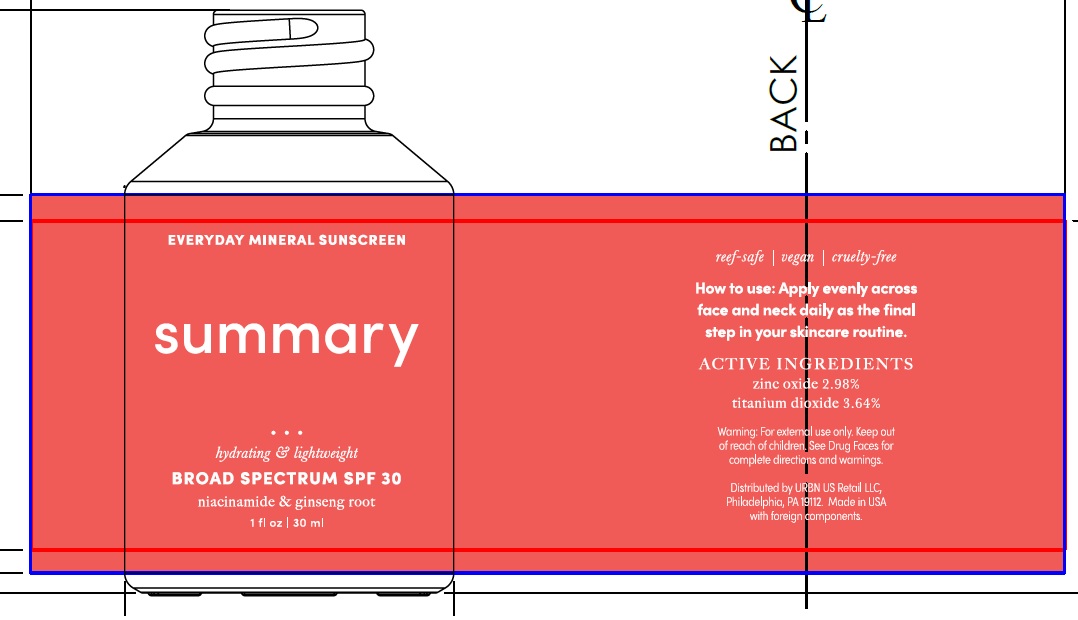
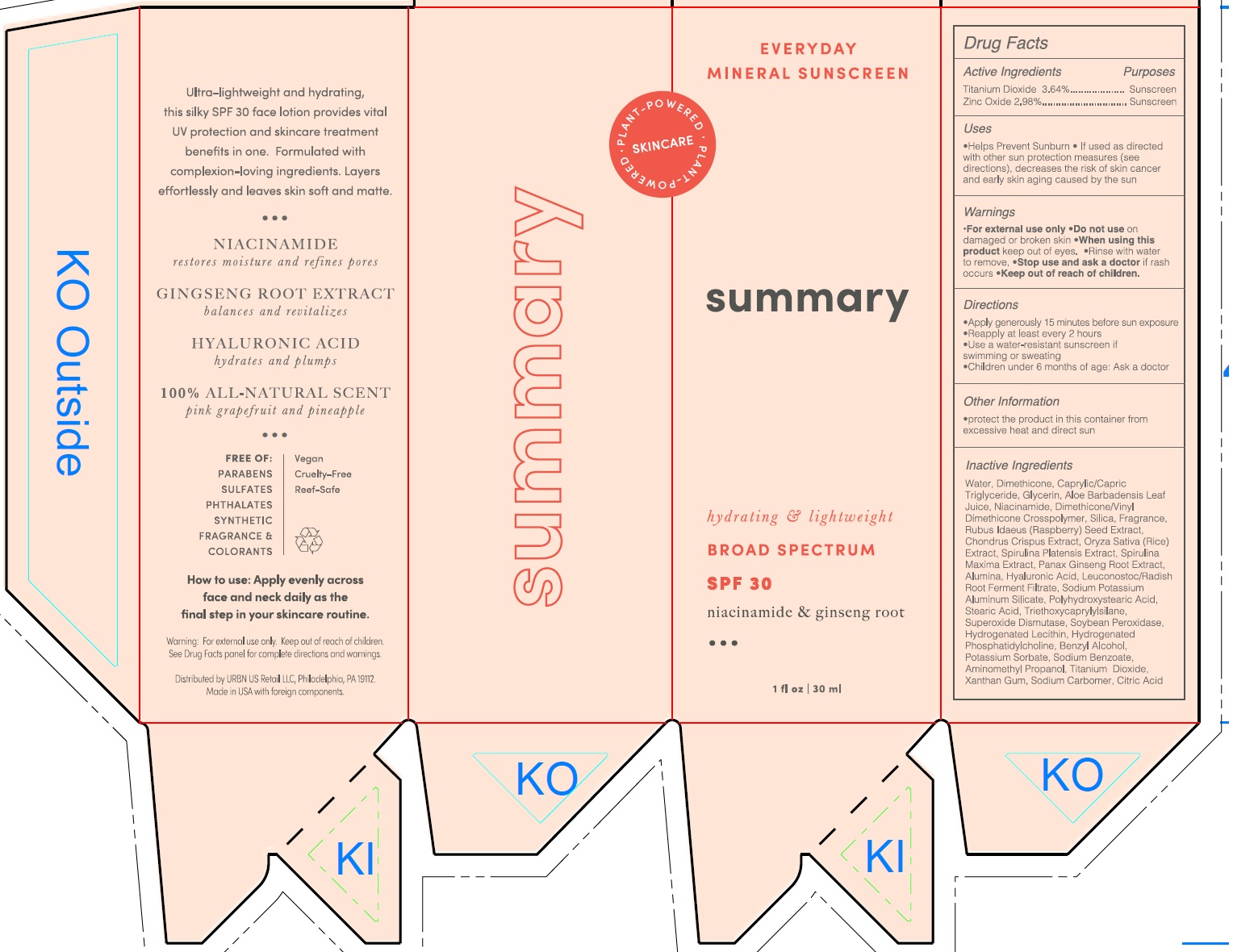
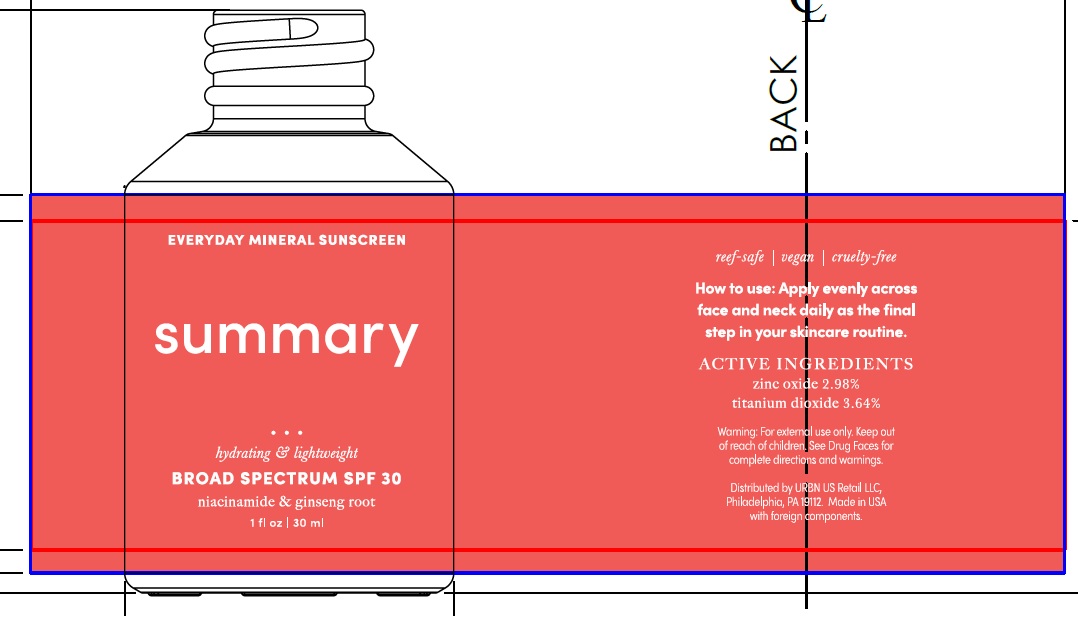
Label: SUMMARY EVERYDAY MINERAL SUNSCREEN BROAD SPECTRUM SPF 30- titanium dioxide, zinc oxide lotion
- NDC Code(s): 14268-120-30
- Packager: ENGLEWOOD LAB, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
- Directions
- Other Information
-
Inactive Ingredients
Water, Diemthicone, Caprylic/Capric Triglyceride, Glycerin, Aloe Barbadensis Leaf Juice, Niacinamide, Dimethicone/Vinyl Dimethicone Crosspolymer, Silica, Fragrance, Rubus Idaeus(Raspberry) Seed Extract, Chondrus Crispus Extract, Oryza Sativa (Rice) Extract, Spirulina Platensis Extract, Spirulina Maxima Extract, Panax Ginseng Root Extract, Alumina, Hyaluronic Acid, Leuconostoc/Radish Root Ferment Filtrate, Sodium Potassium Aluminum Silicate, Polyhydroxystearic Acid, Stearic Acid, Triethoxycaprylylsilane, Superoxide Dismutase, Soybean Peroxidase, Hydrogenated Lecithin, Hydrogenated Phosphatidylcholine, Benzyl Alcohol, Potassium Sorbate, Sodium Benzoate, Aminomethyl Propanol, Titanium Dioxide, Xanthan Gum, Sodium Carbomer, Citric Acid
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SUMMARY EVERYDAY MINERAL SUNSCREEN BROAD SPECTRUM SPF 30
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14268-120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 364 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 298 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) NIACINAMIDE (UNII: 25X51I8RD4) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) RUBUS IDAEUS SEED (UNII: M3CL7US2ZG) CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) RICE GERM (UNII: 7N2B70SFEZ) ARTHROSPIRA MAXIMA (UNII: 9K7IG15M0Q) ASIAN GINSENG (UNII: CUQ3A77YXI) ALUMINUM OXIDE (UNII: LMI26O6933) HYALURONIC ACID (UNII: S270N0TRQY) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SUPEROXIDE DISMUTASE (SACCHAROMYCES CEREVISIAE) (UNII: W2T4YRA9AD) BENZYL ALCOHOL (UNII: LKG8494WBH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) XANTHAN GUM (UNII: TTV12P4NEE) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14268-120-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/01/2023 Labeler - ENGLEWOOD LAB, INC. (172198223)