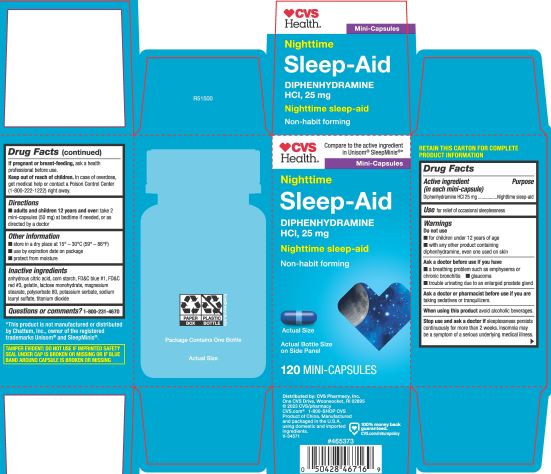
Label: DIPHENHYDRAMINE HYDROCHLORIDE capsule
- NDC Code(s): 51316-347-05, 51316-347-06
- Packager: CVS Pharmacy, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- Use
-
WARNINGS
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- Directions
- Other information
- INACTIVE INGREDIENT
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2023 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
Product of China. Manufactured and packaged in the U.S.A. using domestic and imported ingredients.
V-34571*This product is not manufactured or distributed by Chattem, Inc., owner of the registered trademarks Unisom® and SleepMinis®.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING OR IF BLUE BAND AROUND CAPSULE IS BROKEN OR MISSING.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51316-347 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) GELATIN (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) STARCH, CORN (UNII: O8232NY3SJ) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color blue Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 5347 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-347-05 1 in 1 CARTON 02/02/2023 1 120 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:51316-347-06 365 in 1 BOTTLE; Type 0: Not a Combination Product 04/20/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 02/02/2023 Labeler - CVS Pharmacy, Inc (062312574)