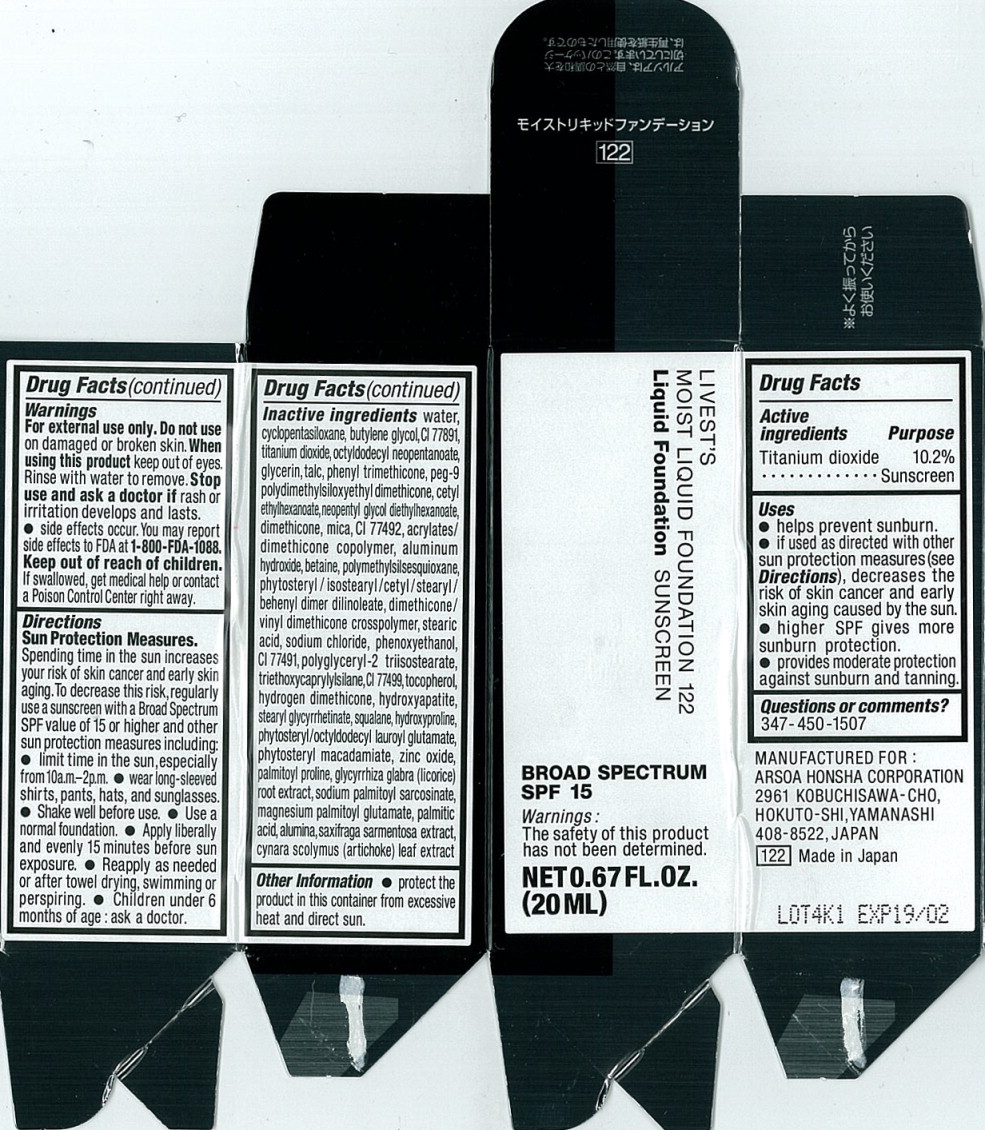
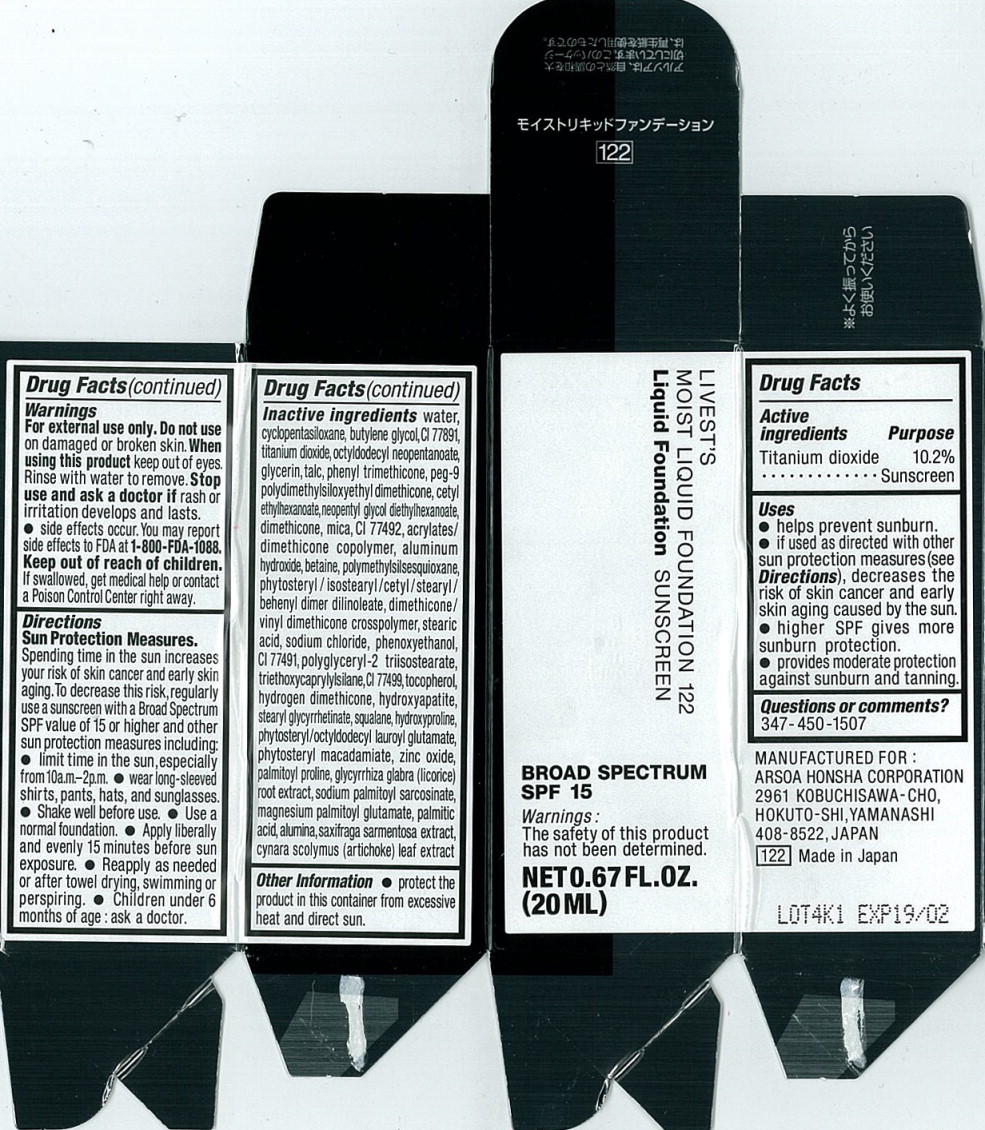
Label: LIVESTS MOIST FOUNDATION 122- titanium dioxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 55655-270-01 - Packager: ARSOA HONSHA CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 9, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn.
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- higher SPF gives more sunburn protection.
- provides moderate protection against sunburn and tanning.
- Warnings
-
Directions
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from10a.m.-2p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
- Shake well before use.
- Use a normal foundation.
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply as needed or after towel drying, swimming or perspiring.
- Children under 6 months of age: ask a doctor.
-
Inactive ingredients
water, cyclopentasiloxane, butylene glycol, Cl 77891, titanium dioxide, octyldodecyl neopentanoate, glycerin, talc, phenyl trimethicone, peg-9 olydimethylsiloxyethyl dimethicone, cetyl ethylhexanoate, neopentyl glycol diethylhexanoate, dimethicone, mica, Cl 77492, acrylates/dimethicone copolymer, aluminum hydroxide, betaine, polymethylsilsesquioxane, phytosteryl/isostearyl/cetyl/stearyl/behenyl dimer dilinoleate, dimethicone/vinyl dimethicone crosspolymer, stearic acid, sodium chloride, phenoxyethanol, Cl 77491, polyglyceryl-2 triisostearate, triethoxycaprylylsilane, Cl 77499, tocopherol, hydrogen dimethicone, hydroxyapatite, stearyl glycyrrhetinate, squalane, hydroxyproline, phytosteryl/octyldodecyl lauroyl glutamate, phytosteryl macadamlate, zinc oxide, palmitoyl proline, glycyrrhiza glabra (licorice) root extract, sodium palmitoyl sarcosinate, magnesium palmitoyl glutamate, palmitic acid, alumina, saxifraga sarmentosa extract, cynara scolymus (artichoke) leaf extract
- Other Information
- Principal Display Panel - 20 mL Carton Label
-
INGREDIENTS AND APPEARANCE
LIVESTS MOIST FOUNDATION 122
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55655-270 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium dioxide (UNII: 15FIX9V2JP) (Titanium dioxide - UNII:15FIX9V2JP) Titanium dioxide 2.31 g in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TALC (UNII: 7SEV7J4R1U) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) GLYCERIN (UNII: PDC6A3C0OX) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIETHYLHEXANOATE (UNII: U68ZV6W62C) DIMETHICONE (UNII: 92RU3N3Y1O) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BETAINE (UNII: 3SCV180C9W) PHYTOSTERYL/ISOSTEARYL/CETYL/STEARYL/BEHENYL DIMER DILINOLEATE (UNII: 8N725H4EFN) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-2 TRIISOSTEARATE (UNII: 68DUY2D39A) FERRIC OXIDE RED (UNII: 1K09F3G675) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TOCOPHEROL (UNII: R0ZB2556P8) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) HYDROXYPROLINE (UNII: RMB44WO89X) STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) SQUALANE (UNII: GW89575KF9) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE (UNII: 65954KGO9Q) PHYTOSTERYL MACADAMIATE (UNII: 233VSF903M) ZINC OXIDE (UNII: SOI2LOH54Z) PALMITOYL PROLINE (UNII: I49727TDYF) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) SODIUM PALMITOYL SARCOSINATE (UNII: 7297LY09YF) MAGNESIUM PALMITOYL GLUTAMATE (UNII: DH37YM1F48) PALMITIC ACID (UNII: 2V16EO95H1) ALUMINUM OXIDE (UNII: LMI26O6933) SAXIFRAGA STOLONIFERA LEAF (UNII: O3TMV4903H) CYNARA SCOLYMUS LEAF (UNII: B71UA545DE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55655-270-01 1 in 1 BOX 12/09/2016 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/09/2016 Labeler - ARSOA HONSHA CORPORATION (694245452) Establishment Name Address ID/FEI Business Operations NIPPON SHIKIZAI, INC 694869178 ANALYSIS(55655-270) , LABEL(55655-270) , MANUFACTURE(55655-270) , PACK(55655-270) , PARTICLE SIZE REDUCTION(55655-270) , RELABEL(55655-270) , REPACK(55655-270) , STERILIZE(55655-270)