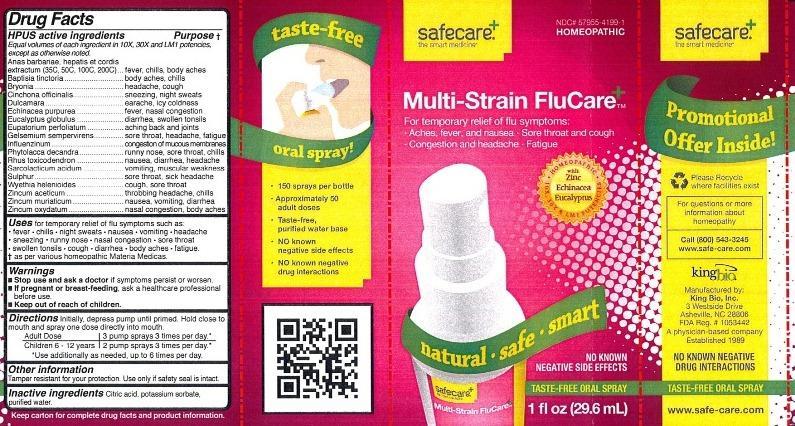
Label: MULTI-STRAIN FLUCARE- anas barbariae, hepatis et cordis extractum, baptisia tinctoria, bryonia, cinchona officinalis, dulcamara, echinacea purpurea, eucalyptus globulus, eupatorium perfoliatum, gelsemium sempervirens, influenzinum, phytolacca decandra, rhus toxicodendron, sarcolacticum acidum, sulphur, wyethia helenioides, zincum aceticum, zincum muriaticum, zincum oxydatum liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-4199-1 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 17, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredients
HPUS active ingredients
Equal volumes of each ingredient in 10X, 30X and LM1 potencies, except as otherwise noted. Anas barbariae, hepatis et cordis extractum (35C, 50C, 100C, 200C)
Anas barbariae, hepatis et cordis extractum, Baptisia tinctoria, Bryonia, Cinchona officinalis, Dulcamara, Echinacea purpurea, Eucalyptus globulus, Eupatorium perfoliatum, Gelsemium sempervirens, Influenzinum, Phytolacca decandra, Rhus toxicodendron, Sarcolacticum acidum, Sulphur, Wyethia helenioides, Zincum aceticum, Zincum muriaticum, Zincum oxydatum.
- Inactive Ingredients
-
Purpose
Drug Facts
HPUS active ingredients Purpose
Equal volumes of each ingredient in 10X, 30X and LM1 potencies, except as otherwise noted.
Anas barbariae, hepatis er cordis extractum (35C, 50C, 100C, 200C)..fever, chills, body aches
Baptisia tinctoria................................................................................body aches, chills
Bryonia..............................................................................................headache, cough
Cinchona officinalis............................................................................sneezing, night sweats
Dulcamara.........................................................................................earache, icy coldness
Echinacea purpurea...........................................................................fever, nasal congestion
Eucalyptus globulus...........................................................................diarrhea, swollen tonsils
Eupatorium perfoliatum......................................................................aching back and joints
Gelsemium sempervirens...................................................................sore throat, headache, fatigue
Influenzinum......................................................................................congestion of mucous membranes
Phytolacca decandra..........................................................................runny nose, sore throat, chills
Rhus toxicodendron...........................................................................nausea, diarrhea, headache
Sarcolacticum acidum.........................................................................vomiting, muscular weakness
Sulphur..............................................................................................sore throat, sick headache
Wyethia helenioides...........................................................................cough, sore throat
Zincum aceticum.................................................................................throbbing headache, chills
Zincum muriaticum..............................................................................nausea, vomiting, diarrhea
Zincum oxydatum................................................................................nasal congestion, body aches
- Dosage and Administration
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Indications and Usage
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MULTI-STRAIN FLUCARE
anas barbariae, hepatis et cordis extractum, baptisia tinctoria, bryonia, cinchona officinalis, dulcamara, echinacea purpurea, eucalyptus globulus, eupatorium perfoliatum, gelsemium sempervirens, influenzinum, phytolacca decandra, rhus toxicodendron, sarcolacticum acidum, sulphur, wyethia helenioides, zincum aceticum, zincum muriaticum, zincum oxydatum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-4199 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 35 [hp_C] in 29.6 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 10 [hp_X] in 29.6 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 10 [hp_X] in 29.6 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 10 [hp_X] in 29.6 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 10 [hp_X] in 29.6 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 10 [hp_X] in 29.6 mL EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 10 [hp_X] in 29.6 mL EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 10 [hp_X] in 29.6 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 10 [hp_X] in 29.6 mL INFLUENZA A VIRUS (UNII: R9HH0NDE2E) (INFLUENZA A VIRUS - UNII:R9HH0NDE2E) INFLUENZA A VIRUS 10 [hp_X] in 29.6 mL INFLUENZA B VIRUS (UNII: 1314JZ2X6W) (INFLUENZA B VIRUS - UNII:1314JZ2X6W) INFLUENZA B VIRUS 10 [hp_X] in 29.6 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 10 [hp_X] in 29.6 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 10 [hp_X] in 29.6 mL LACTIC ACID, L- (UNII: F9S9FFU82N) (LACTIC ACID, L- - UNII:F9S9FFU82N) LACTIC ACID, L- 10 [hp_X] in 29.6 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 10 [hp_X] in 29.6 mL WYETHIA HELENIOIDES ROOT (UNII: J10PD1AQ0N) (WYETHIA HELENIOIDES ROOT - UNII:J10PD1AQ0N) WYETHIA HELENIOIDES ROOT 10 [hp_X] in 29.6 mL ZINC ACETATE ANHYDROUS (UNII: H2ZEY72PME) (ZINC CATION - UNII:13S1S8SF37) ZINC ACETATE ANHYDROUS 10 [hp_X] in 29.6 mL ZINC CHLORIDE (UNII: 86Q357L16B) (ZINC CATION - UNII:13S1S8SF37) ZINC CHLORIDE 10 [hp_X] in 29.6 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 10 [hp_X] in 29.6 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-4199-1 1 in 1 CARTON 1 29.6 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/27/2012 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 api manufacture(57955-4199)