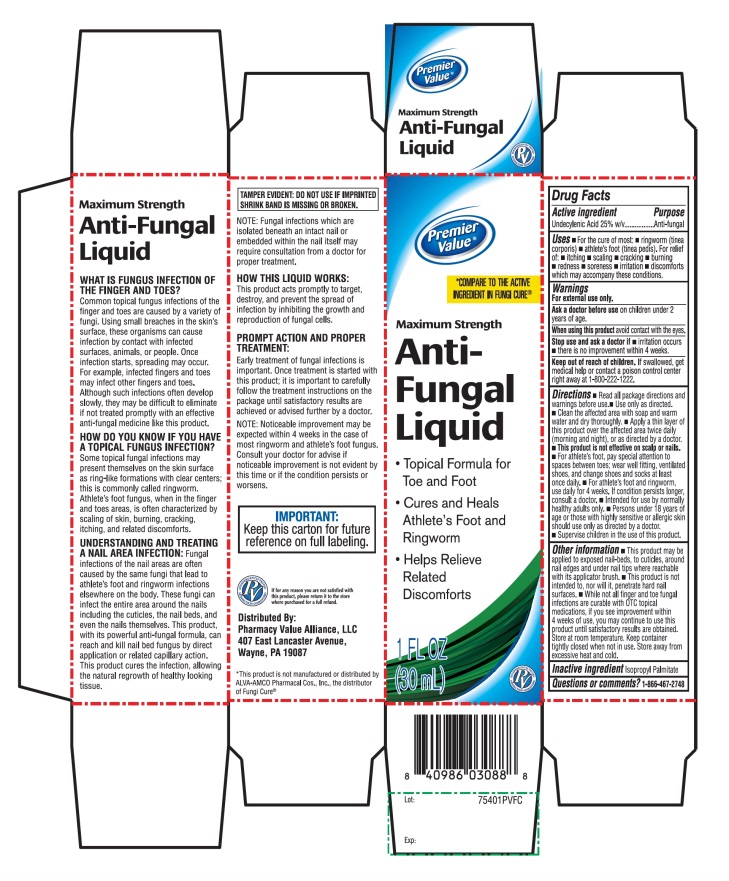
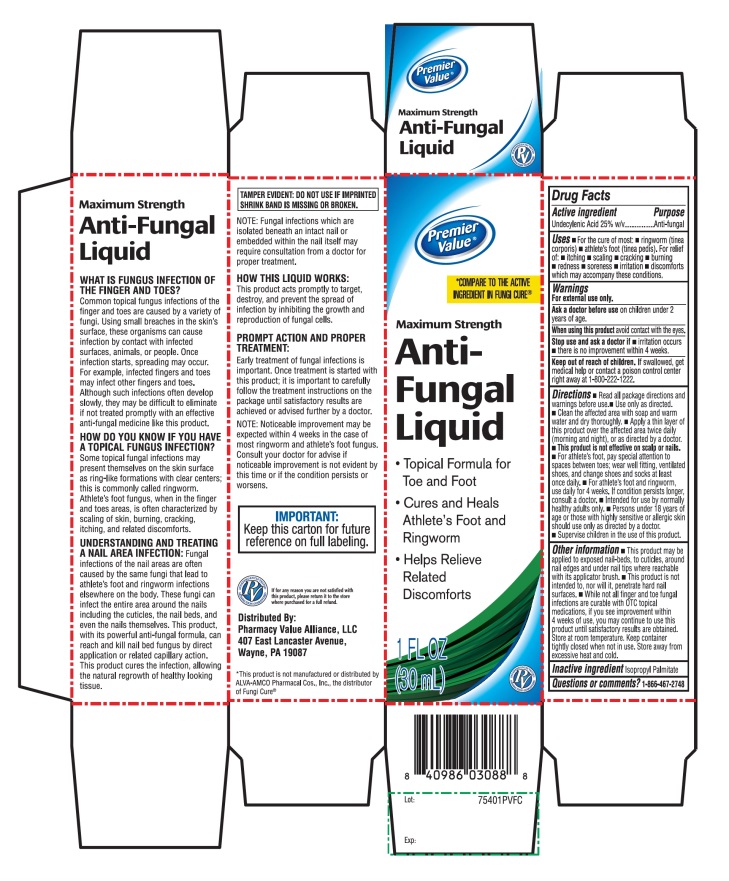
Label: PREMIER VALUE ANTIFUNGAL MAXIMUM STRENGTH- undecylenic acid liquid
- NDC Code(s): 68016-474-00
- Packager: PHARMACY VALUE ALLIANCE, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
Read all warnings and directions.
- ▪
- Read all package directions and warnings before use
- ▪
- Use only as directed
- ▪
- clean the affected area with soap and warm water and dry thoroughly
- ▪
- Apply a thin layer of this product over affected area twice daily (morning and night) or as directed by a doctor.
- ▪
- The brush applicator allows for easy application under nails and surrounding cuticle area.
- ▪
- wear well-fitting, ventilated shoes, and change shoes and socks at least once daily
- ▪
- this product is not effective on the scalp or nails
- ▪
- for athlete's foot: pay special attention to spaces between toes; wear well fitting, ventilated shoes, and change shoes and socks at least once daily.
- ▪
- For athlete's foot and ringworm, use daily for 4 weeks. If conditions persists longer, consult a doctor
- ▪
- Intended for use by normally healthy adults only
- ▪
- Persons under 18 years of age or those with highly sensitive or allergic skin should use only as directed by a doctor
- ▪
- Supervise children in the use of this product
-
Other information
- •
- This product may be applied to exposed nail-beds to cuticles, around nail edges and under nail tips where reachable with its applicator brush
- •
- This product is not intended to, nor will it, penetrate hard nail surfaces
- •
- While not all finger and toe fungal infections are curable with OTC topical medications, if you see improvement within 4 weeks of use, you may continue to use this product until satisfactory results are obtained.
- •
- store at room temperature. Keep the container tightly closed when not in use.
- •
- Store away from excessive heat and cold.
- Inactive ingredient
- Questions?
-
Principal Display Panel
NDC 68016-474-00
COMPARE TO THE ACTIVE INGREDIENT FUNGICURE®
MAXIMUM STRENGTH
Anti-Fungal Liquid- •
- Topical formula for toe and foot
- •
- Cures and Heals athlete’s foot fungus and ringworm
- •
- Helps relieves related discomforts
1 FL OZ (30mL)
IMPORTANT: KEEP this carton for future reference on full labeling
Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087
*This product is not manufactured or distributed by ALVA-AMCO Pharmacal Cos., Inc., the distributor of Fungi Cure®
-
INGREDIENTS AND APPEARANCE
PREMIER VALUE ANTIFUNGAL MAXIMUM STRENGTH
undecylenic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-474 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 25 mg in 30 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-474-00 30 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 04/10/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 04/10/2019 Labeler - PHARMACY VALUE ALLIANCE, LLC (101668460)