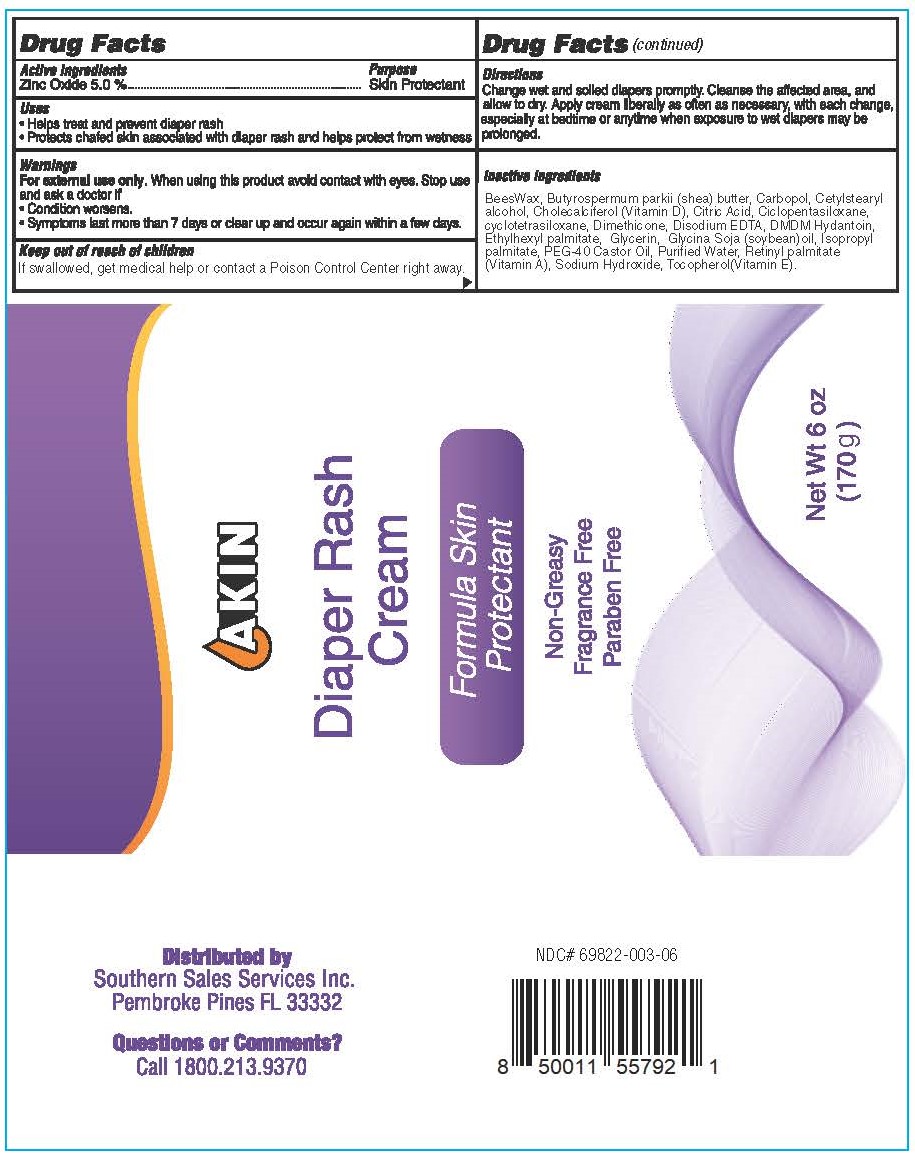
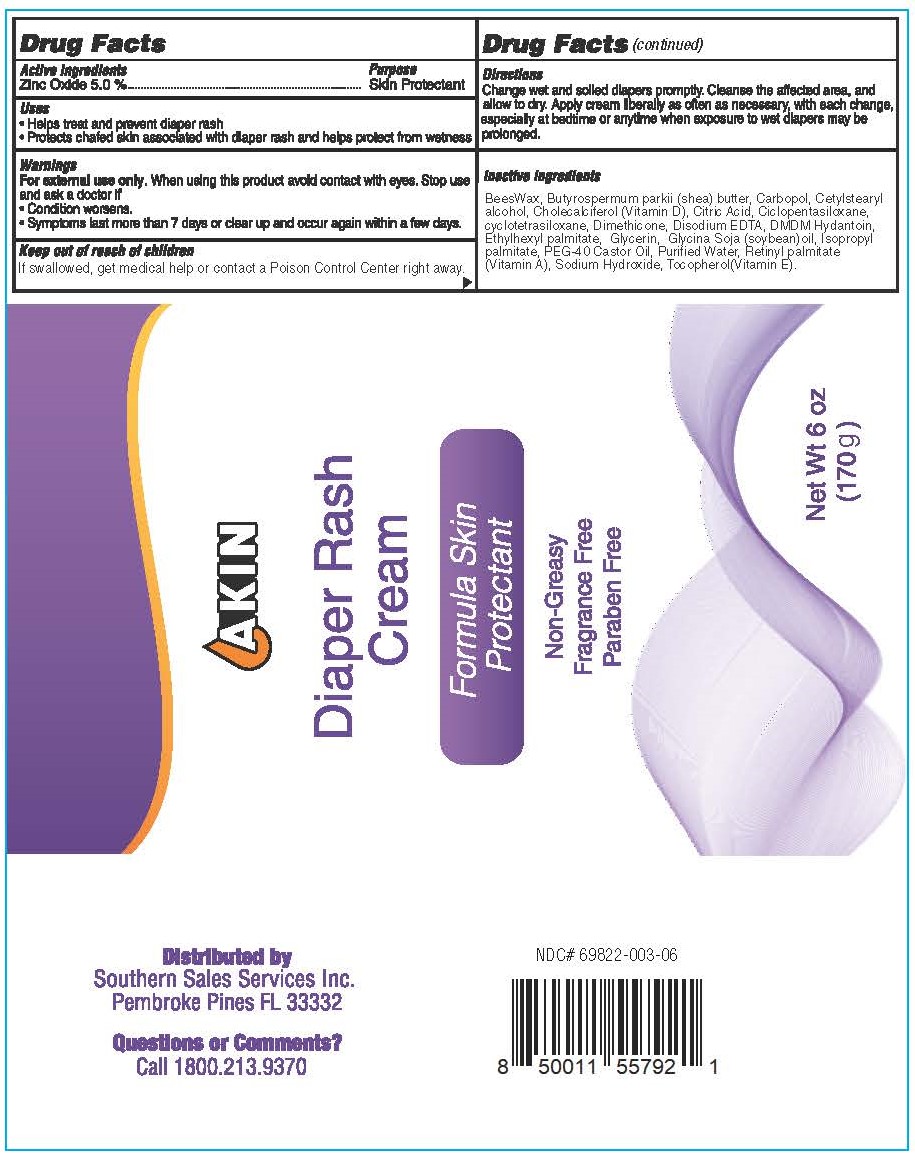
Label: AKIN DIAPER RASH- zinc oxide cream
- NDC Code(s): 69822-003-06
- Packager: Southern Sales & Service, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- Keep out of reach of children
- Directions
-
Inactive ingredients
BeesWax, Butyrospermum parkii (shea) butter, Carbopol, Cetylstearyl alcohol, Cholecalciferol (Vitamin D), Citric Acid, Ciclopentasiloxane, cyclotetrasiloxane, Dimethicone, Disodium EDTA, DMDM Hydantoin, Ethylhexyl palmitate, Glycerin, Glycina Soja (soybean)oil, Isopropyl palmitate, PEG-40 Castor Oil, Purified Water, Retinyl palmitate (Vitamin A), Sodium Hydroxide, Tocopherol(Vitamin E).
- Akin Diaper Rash Cream 170 g
-
INGREDIENTS AND APPEARANCE
AKIN DIAPER RASH
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69822-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 50 mg in 1 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) SHEA BUTTER (UNII: K49155WL9Y) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CHOLECALCIFEROL (UNII: 1C6V77QF41) CITRIC ACID ACETATE (UNII: DSO12WL7AU) CYCLOMETHICONE 4 (UNII: CZ227117JE) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) DMDM HYDANTOIN (UNII: BYR0546TOW) ETHYLHEXYL PALMITATE (UNII: 2865993309) GLYCERIN (UNII: PDC6A3C0OX) SOYBEAN OIL (UNII: 241ATL177A) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) PEG-40 CASTOR OIL (UNII: 4ERD2076EF) WATER (UNII: 059QF0KO0R) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SODIUM HYDROXIDE (UNII: 55X04QC32I) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69822-003-06 170 g in 1 TUBE; Type 0: Not a Combination Product 04/25/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 04/25/2019 Labeler - Southern Sales & Service, Inc. (013114906) Registrant - Southern Sales & Service, Inc. (013114906)