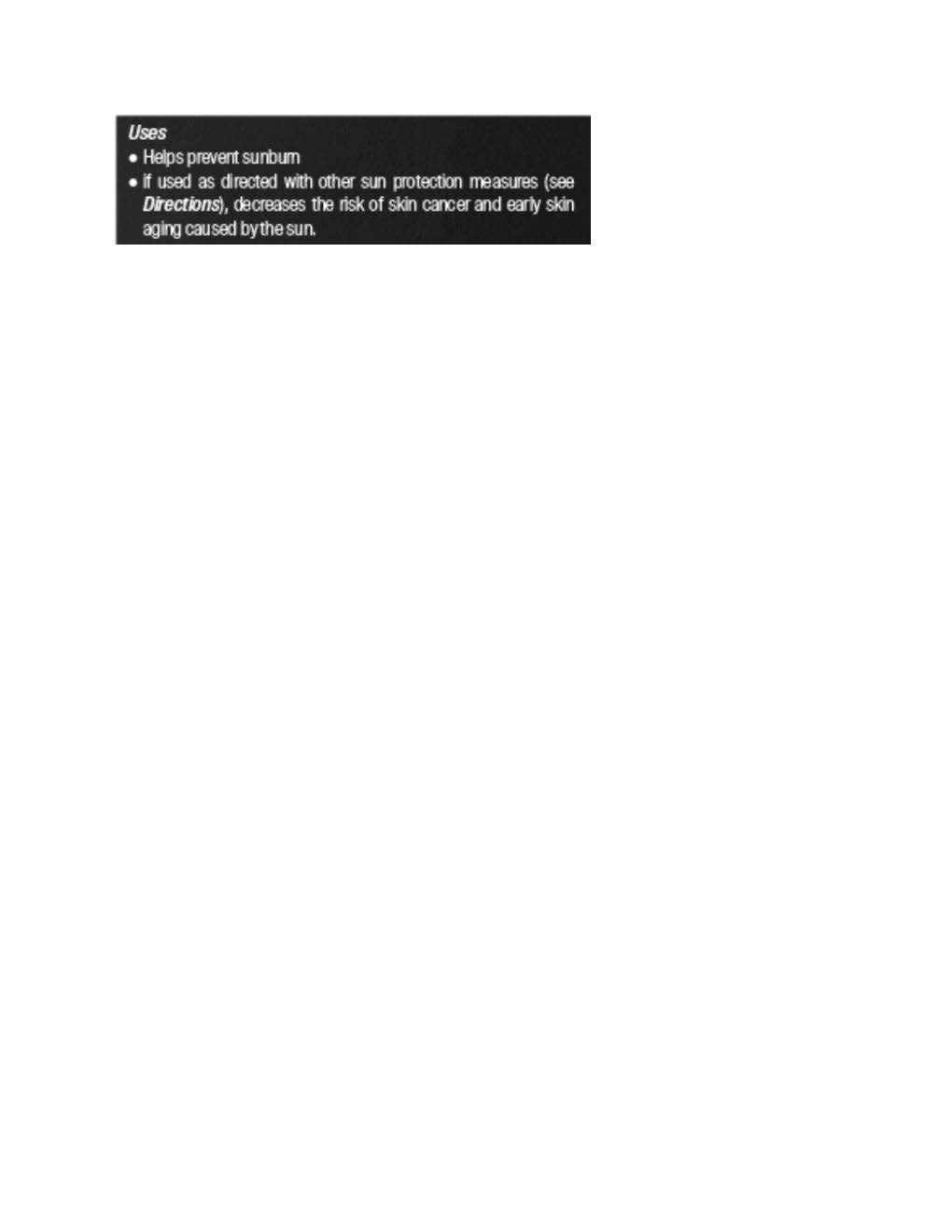
Label: PAVISE DYNAMIC AGE DEFENSE- zinc oxide lotion
-
NDC Code(s):
83100-1720-1,
83100-1720-2,
83100-1720-3,
83100-1720-4, view more83100-1720-5
- Packager: B.A.I BIOSCIENCES, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- pavise Dynamic Age Defense
- pavise Dynamic Age Defense
- pavise Dynamic Age Defense
- pavise Dynamic Age Defense
- pavise Dynamic Age Defense
- pavise Dynamic Age Defense
- pavise Dynamic Age Defense
- pavise Dynamic Age Defense
-
INGREDIENTS AND APPEARANCE
PAVISE DYNAMIC AGE DEFENSE
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83100-1720 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 174 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) XANTHAN GUM (UNII: TTV12P4NEE) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) ASTAXANTHIN (UNII: 8XPW32PR7I) ISOSTEARIC ACID (UNII: X33R8U0062) PINUS PINASTER WOOD (UNII: 0L1DQ35D6G) PROPANEDIOL (UNII: 5965N8W85T) CETYL ALCOHOL (UNII: 936JST6JCN) OCTYLDODECANOL (UNII: 461N1O614Y) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILYBUM MARIANUM SEED (UNII: U946SH95EE) TRANEXAMIC ACID (UNII: 6T84R30KC1) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) WATER (UNII: 059QF0KO0R) NIACINAMIDE (UNII: 25X51I8RD4) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) DISODIUM ETHYLENEDIAMINEDIACETATE (UNII: EQL53S5L0F) POLYGLYCERYL-3 DISTEARATE (UNII: ZI1LK470XV) ARGANIA SPINOSA SEED (UNII: 8H7X7XB54H) SQUALANE (UNII: GW89575KF9) TRIACONTANYL PVP (WP-660) (UNII: N0SS3Q238D) DIAMOND (UNII: 6GRV67N0U2) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83100-1720-3 1 in 1 CARTON 02/17/2023 1 NDC:83100-1720-2 1 in 1 BOX 1 NDC:83100-1720-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:83100-1720-5 2 in 1 CARTON 08/25/2023 2 NDC:83100-1720-4 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/17/2023 Labeler - B.A.I BIOSCIENCES, INC (083612207) Registrant - Nanophase Technologies Corporation (623502044) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 050383046 api manufacture(83100-1720) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 118812921 pack(83100-1720) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 623502044 api manufacture(83100-1720) , manufacture(83100-1720)