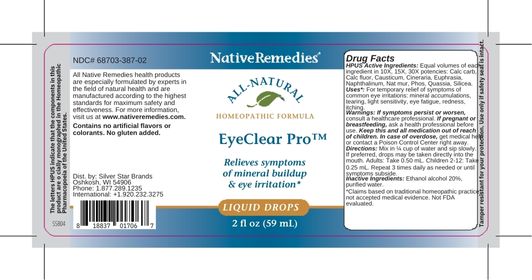
Label: EYECLEAR PRO- calc carb, calc fluor, causticum, cineraria, euphrasia, naphthalinum, nat mur, phos, quassia, silicea liquid
- NDC Code(s): 68703-387-02
- Packager: Silver Star Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HPUS Active Ingredients
HPUS Active Ingredients: Equal volumes of each ingredient in 10X, 15X, 30X potencies: Calc carb, Calc fluor, Causticum, Cineraria, Euphrasia, Naphthalinum, Nat mur, Phos, Quassia, Silicea.
The letters UPUS indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
- Uses*
- Warnings
- Directions
- OTHER SAFETY INFORMATION
- Inactive Ingredients
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EYECLEAR PRO
calc carb, calc fluor, causticum, cineraria, euphrasia, naphthalinum, nat mur, phos, quassia, silicea liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68703-387 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPHTHALENE (UNII: 2166IN72UN) (NAPHTHALENE - UNII:2166IN72UN) NAPHTHALENE 30 [hp_X] in 59 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 30 [hp_X] in 59 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 30 [hp_X] in 59 mL SENECIO VULGARIS (UNII: W6MU8IYV12) (SENECIO VULGARIS - UNII:W6MU8IYV12) SENECIO VULGARIS 30 [hp_X] in 59 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_X] in 59 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_X] in 59 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_X] in 59 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_X] in 59 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 30 [hp_X] in 59 mL QUASSIA AMARA WOOD (UNII: S5249Q85HW) (QUASSIA AMARA WOOD - UNII:S5249Q85HW) QUASSIA AMARA WOOD 30 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68703-387-02 59 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 03/13/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/13/2023 Labeler - Silver Star Brands (006070379) Registrant - Silver Star Brands (006070379) Establishment Name Address ID/FEI Business Operations OHM Pharma 030572478 manufacture(68703-387)