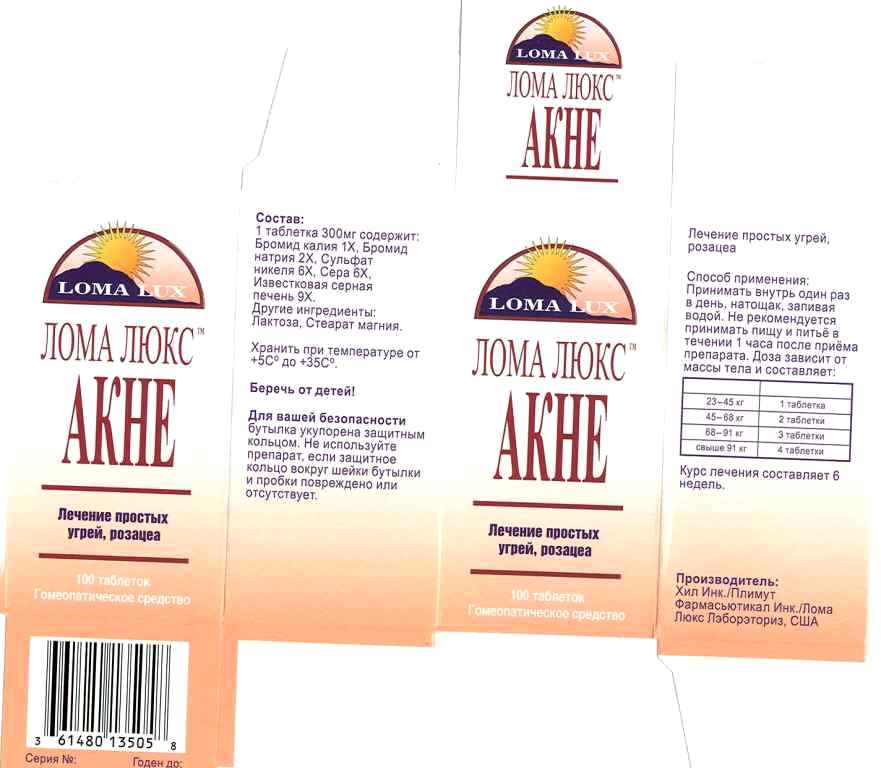
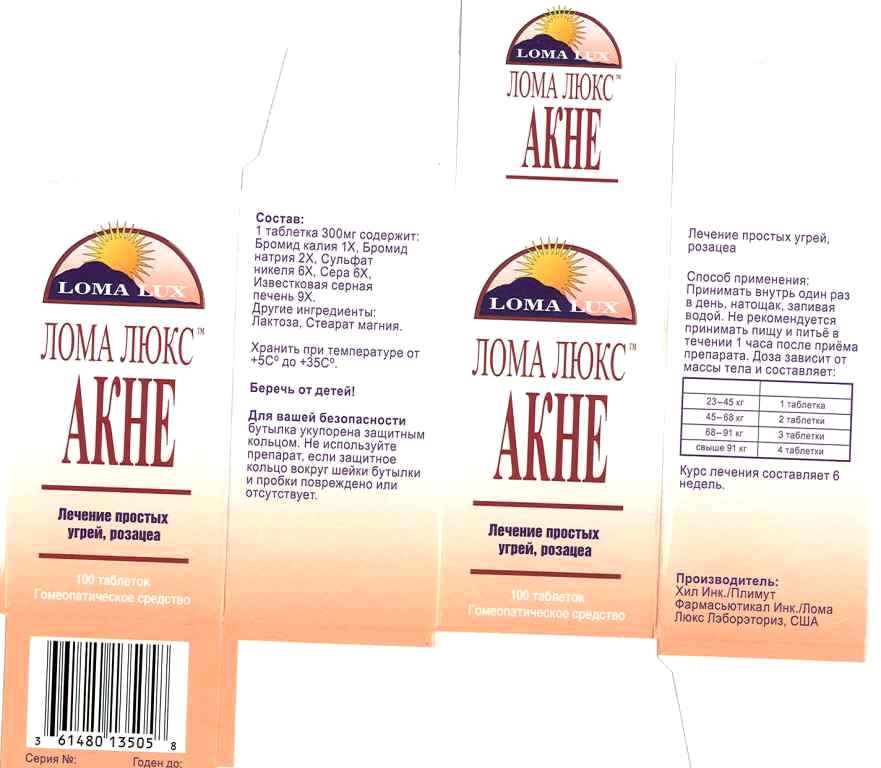
Label: ACNE- kali bromatum, natrum bromatum, niccolum sulphuricum, sulphur, hepar sulphurs calcareum, tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 57520-0711-1, 57520-0711-2, 57520-0711-3 - Packager: Apotheca Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 29, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
WARNINGS: If symptoms persist or worsen, contact a physician.
If pregnant or nursing, use only under the advice and supervision of a physician.
Keep our of the reach of children.
Safety Sealed for your protection. Do not use if imprinted seal around bottle neck and cap is missing or broken.
CAUTION: Use only as directed.
Do not give to children under six years old or use in the presence of kidney disease.
If skin rash appears, or if nervous symptoms persist, recur frequently, or are unusual, discontinue use and consult a physician.
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACNE
kali bromatum, natrum bromatum, niccolum sulphuricum, sulphur, hepar sulphurs calcareum, tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57520-0711 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM BROMIDE (UNII: OSD78555ZM) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM BROMIDE 1 [hp_X] SODIUM BROMIDE (UNII: LC1V549NOM) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM BROMIDE 2 [hp_X] NICKEL SULFATE HEXAHYDRATE (UNII: JC9WZ4FK68) (NICKEL - UNII:7OV03QG267) NICKEL SULFATE HEXAHYDRATE 6 [hp_X] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_X] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFIDE 9 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (off white) Score no score Shape ROUND (cylindrical) Size 9mm Flavor Imprint Code LL; Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57520-0711-3 1 in 1 CARTON 1 NDC:57520-0711-2 1 in 1 CARTON 1 NDC:57520-0711-1 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/29/2011 Labeler - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture