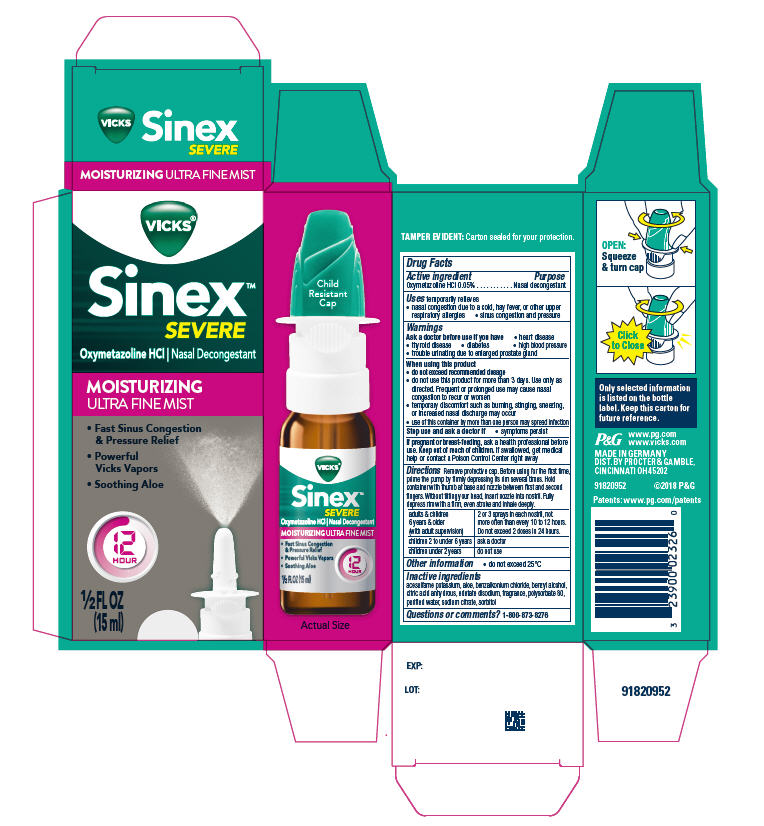
Label: VICKS SINEX SEVERE MOISTURIZING ULTRA FINE MIST- oxymetazoline hydrochloride spray
- NDC Code(s): 64336-015-01
- Packager: Procter & Gamble Manufacturing GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
When using this product
- do not exceed recommended dosage
- do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen
- temporary discomfort such as burning, stinging, sneezing, or increased nasal discharge may occur
- use of this container by more than one person may spread infection
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
-
Directions
Remove protective cap. Before using for the first time, prime the pump by firmly depressing its rim several times. Hold container with thumb at base and nozzle between first and second fingers. Without tilting your head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and inhale deeply.
adults & children 6 yrs. & older (with adult supervision) 2 or 3 sprays in each nostril, not more often than every 10 to 12 hours. Do not exceed 2 doses in 24 hours. children 2 to under 6 yrs. ask a doctor children under 2 yrs. do not use - Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 15 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
VICKS SINEX SEVERE MOISTURIZING ULTRA FINE MIST
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64336-015 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.05 g in 1 mL Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) ALOE (UNII: V5VD430YW9) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Product Characteristics Color white (Clear) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64336-015-01 1 in 1 CARTON 07/10/2018 1 15 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/10/2018 Labeler - Procter & Gamble Manufacturing GmbH (333608813)