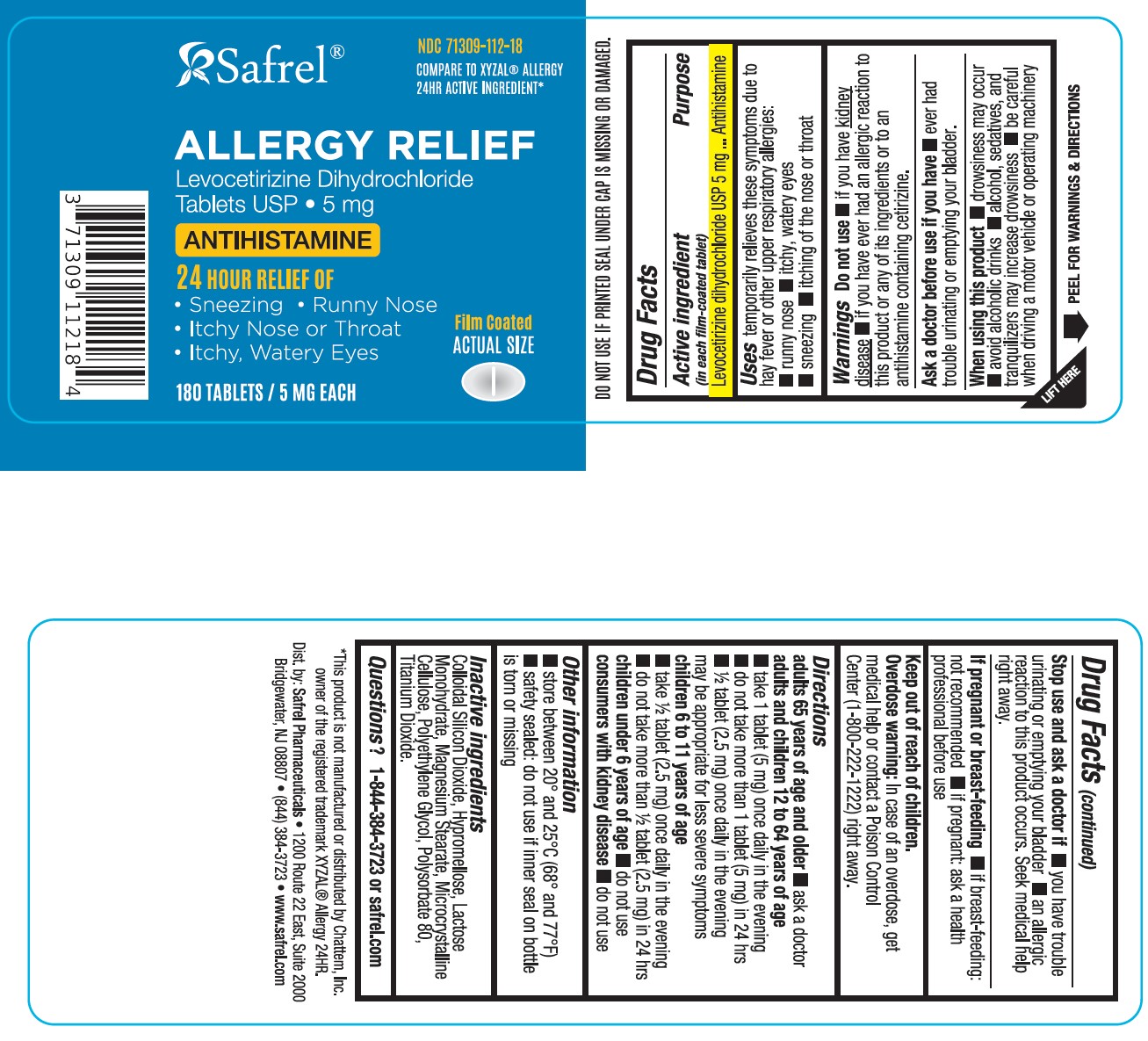
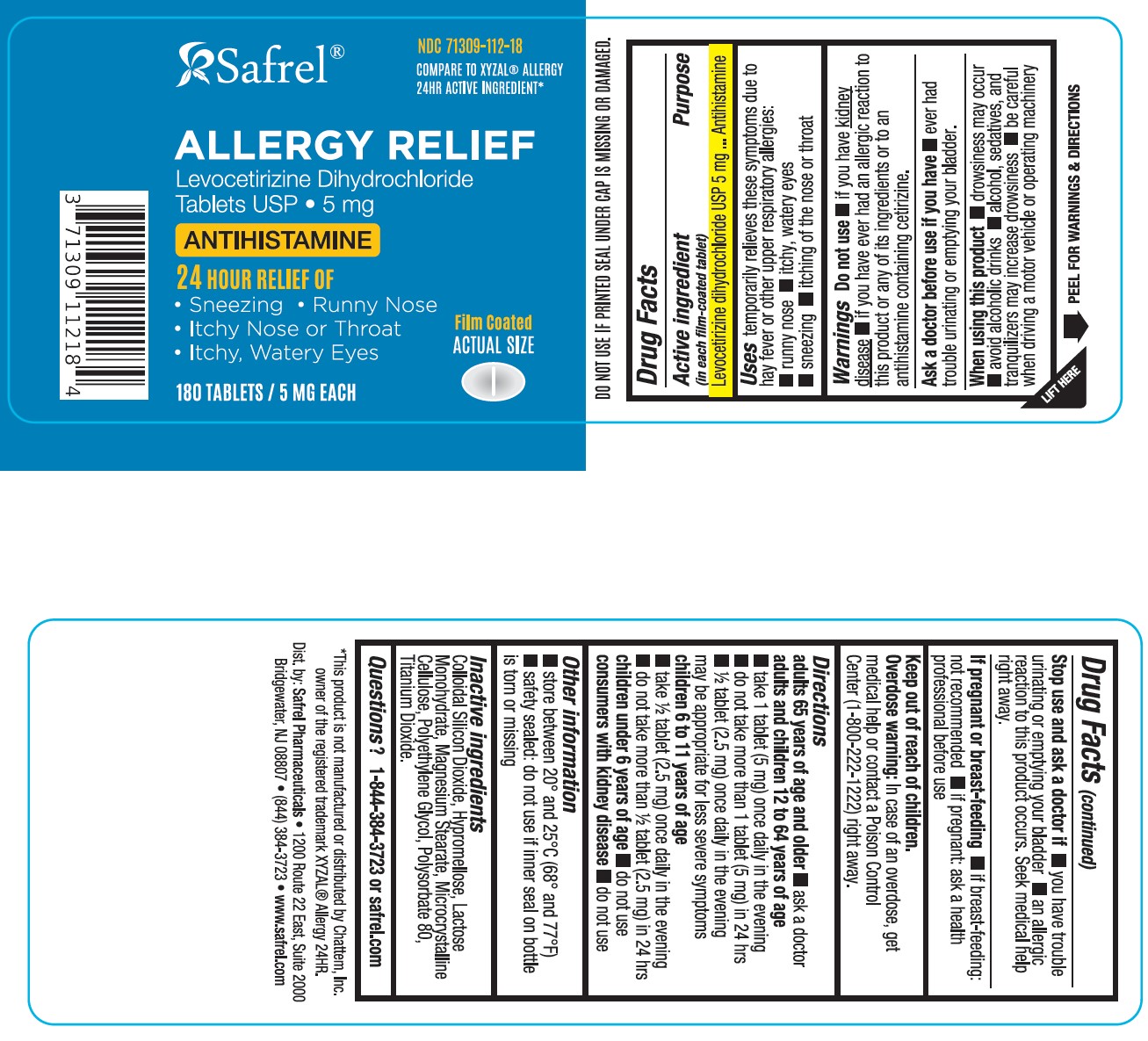
Label: LEVOCETIRIZINE DIHYDROCHLORIDE tablet, film coated
- NDC Code(s): 71309-112-18
- Packager: Safrel Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- DO NOT USE
- ASK A DOCTOR BEFORE USE IF YOU HAVE
- WHEN USING THIS PRODUCT
- STOP USE AND ASK A DOCTOR IF
- IF PREGNANT OR BREAST-FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
adults 65 years of age and older
- ask a doctor
adults and children 12 to 64 years of age
- take 1 tablet (5 mg) once daily in the evening
- do not take more than 1 tablet (5 mg) in 24 hours
- ½ tablet (2.5 mg) once daily in the evening may be appropriate for less severe symptoms
children 6 to 11 years of age
- take ½ tablet (2.5 mg) once daily in the evening
- do not take more than ½ tablet (2.5 mg) in 24 hours
children under 6 years of age
- do not use
consumers with kidney disease
- do not use
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS or COMMENTS?
-
PRINCIPAL DISPLAY PANEL
Levocetirizine Dihydrochloride Tablets USP 5 mg-180 Tablets
Compare to XYZAL ® Allergy 24HR Active Ingredient*Allergy Relief
Levocetirizine Dihydrochloride
Tablets, USP5 mg
Antihistamine
24 HOUR Relief of
- Sneezing
- Runny Nose
- Itchy Nose or Throat
- Itchy, Watery Eyes
Original Prescription Strength
180 TABLETS
-
INGREDIENTS AND APPEARANCE
LEVOCETIRIZINE DIHYDROCHLORIDE
levocetirizine dihydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71309-112 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOCETIRIZINE DIHYDROCHLORIDE (UNII: SOD6A38AGA) (LEVOCETIRIZINE - UNII:6U5EA9RT2O) LEVOCETIRIZINE DIHYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color white (White to off white) Score 2 pieces Shape OVAL Size 8mm Flavor Imprint Code H;LL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71309-112-18 180 in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213513 10/28/2022 Labeler - Safrel Pharmaceuticals LLC (080566287)