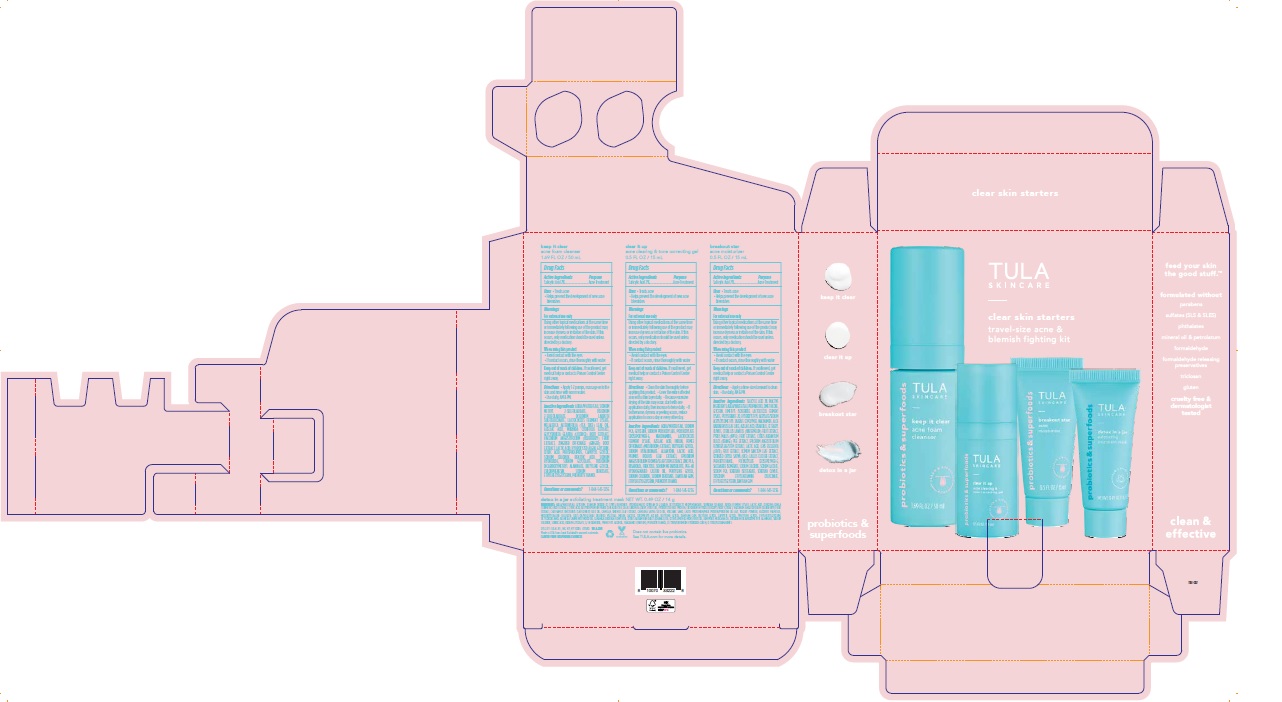
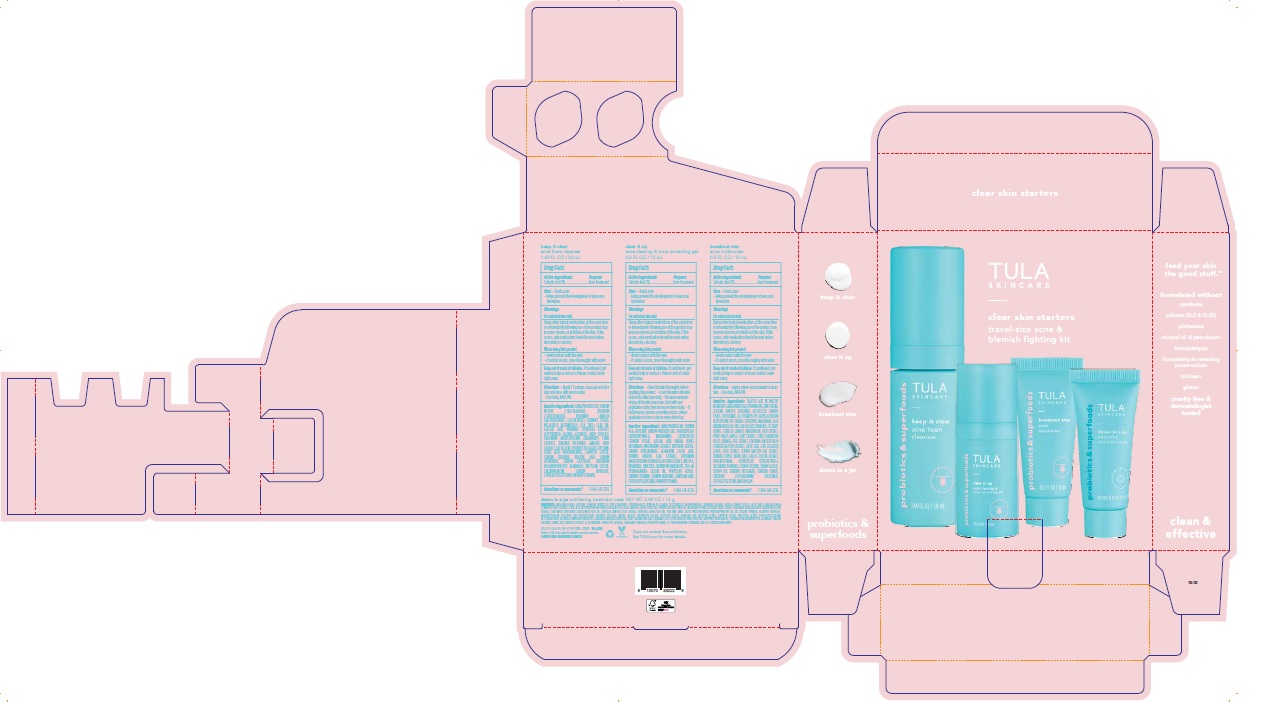
Label: TULA SKINCARE CLEAR SKIN STARTERS TRAVEL-SIZE ACNE AND BLEMISH FIGHTING KIT- acne and blemish fighting kit kit
- NDC Code(s): 72296-906-01
- Packager: TULA Life LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only
Using other topical medications at the same time or
immediately following use of this product may increase
dryness or irritation of the skin. If this occurs, only one
medication should be used unless directed by a doctor.When using this product
• avoid contact with the eyes
• if contact occurs, rinse thoroughly with water - Keep out of reach of children.
-
Directions
• clean the skin thoroughly before applying this product
• cover the entire affected area with a thin layer one to three
times daily
• because excessive drying of the skin may occur, start with
one application daily, then gradually increase to two or three
times daily if needed or as directed by a doctor
• if bothersome dryness or peeling occurs, reduce application
to once a day or every other day -
Inactive ingredients
TULA Skincare Keep it Clear Acne Foam Cleanser:
Aqua/Water/Eau, Sodium Methyl 2-Sulfolaurate, Disodium 2-Sulfolaurate, Disodium Laureth Sulfosuccinate, Lactococcus Ferment Lysate, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Azelaic Acid, Morinda Citrifolia Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Vaccinium Angustifolium (Blueberry) Fruit Extract, Zingiber Officinale (Ginger) Root Extract, Lactic Acid, Hydrolyzed Algin, Glycerin, Citric Acid, Phospholipids, Caprylyl Glycol, Sodium Chloride, Benzoic Acid, Sodium Hydroxide, Sodium Glycolate, Trisodium Dicarboxymethyl Alaninate, Butylene Glycol, Chlorphenesin, Sodium Benzoate, Ethylhexylglycerin, Phenoxyethanol
TULA breakout star oil-free acne moisturizer:
Aqua/Water/Eau, Propanediol, Dimethicone, Glycerin, Dimethyl Isosorbide, Lactococcus Ferment Lysate, Polysorbate 20, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Niacinamide, Aloe Barbadensis Leaf Juice, Azelaic Acid, Bisabolol, Cetearyl Olivate, Cirusllus Lanatus (Watermelon) Fruit Extract, Pyrus Malus (Apple) Fruit Extract, Citrus Aurantium Dulcis (Orange) Peel Extract, Epilobium Angustifolium Flower/Leaf/Stem Extract, Lactic Acid, Lens Esculenta (Lentil) Fruit Extract, Ocimum Sanctum Leaf Extract, Ozonized Oryza Sativa (Rice) Callus Culture Extract, Phenoxyethanol, Polyacrylate Crosspolymer-6, Saccharide Isomerate, Sodium Chloride, Sodium Lactate, Sodium PCA, Sorbitan Isostearate, Sorbitan Olivate, Trisodium Ethylenediamine Disuccinate, Ethylhexylglycerin, Xanthan Gum
TULA acne clearing and tone correcting gel:
Water/Aqua/Eau, Sodium Polyacrylate, Sodium PCA, Glycerin, Polyacrylate Crosspolymer-6, Azelaic Acid, Lactococcus Ferment Lysate, Zinc PCA, Epilobium Angustifolium Flower/Leaf/Stem Extract, Niacinamide, Bisabolol, Allantoin, Sodium Hyaluronate, Lactic Acid, Sodium Benzoate, Sodium Chloride, Sodium Metabisulfite, Ehtylhexylglyceirn, Phenoxyethanol
-
Ingredients List for TULA Skincare Detox in a jar exfoliating treatment mask (Cosmetic component)
INGREDIENTS: AQUA/WATER/EAU, GLYCERIN, TITANIUM DIOXIDE (CI 77891), BENTONITE, HYDROGENATED SOYBEAN OIL, KAOLIN, OCTYLDODECYL NEOPENTANOATE, SORBITAN STEARATE, BIFIDA FERMENT LYSATE, LACTIC ACID, CURCUMA LONGA (TURMERIC) ROOT EXTRACT, CITRIC ACID, BUTYROSPERMUM PARKII (SHEA) BUTTER, OLEA EUROPAEA (OLIVE) FRUIT OIL, HYDROLYZED RICE PROTEIN, CICHORIUM INTYBUS (CHICORY) ROOT EXTRACT, VACCINIUM ANGUSTIFOLIUM (BLUEBERRY) FRUIT EXTRACT, CARTHAMUS TINCTORIUS (SAFFLOWER) SEED OIL, CAMELLIA SINENSIS LEAF EXTRACT, CAMELINA SATIVA SEED OIL, VOLCANIC SAND, LACTIS PROTEINUM/MILK PROTEIN/PROTÉINE DU LAIT, YOGURT POWDER, ASCORBYL PALMITATE, MICROCRYSTALLINE CELLULOSE, OLUS OIL/VEGETABLE OIL/HUILE VÉGÉTALE, INULIN, LACTOSE, TOCOPHERYL ACETATE, BUTYLENE GLYCOL, XANTHAN GUM, DECYLENE GLYCOL, CAPRYLYL GLYCOL, PENTYLENE GLYCOL, ETHYLHEXYLGLYCERIN, OCTYLDODECANOL, BULNESIA SARMIENTOI WOOD OIL, CANANGA ODORATA FLOWER OIL, CITRUS AURANTIUM DULCIS (ORANGE) OIL, CITRUS LIMON (LEMON) FRUIT OIL, JUNIPERUS MEXICANA OIL, TRISODIUM DICARBOXYMETHYL ALANINATE, SODIUM CHLORIDE, SORBIC ACID, SODIUM GLYCOLATE, 1,2 HEXANEDIOL, PHENETHYL ALCOHOL, FRAGRANCE (PARFUM), PHENOXYETHANOL, CI 77289/CHROMIUM HYDROXIDE GREEN, CI 77007/ULTRAMARINES.
- Questions or Comments?
- TULA Skincare Clear Skin Starters Travel-size Acne and Blemish Fighting Kit Artwork
-
INGREDIENTS AND APPEARANCE
TULA SKINCARE CLEAR SKIN STARTERS TRAVEL-SIZE ACNE AND BLEMISH FIGHTING KIT
acne and blemish fighting kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72296-906 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72296-906-01 1 in 1 CARTON 02/14/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 14 g in 100 Part 2 1 BOTTLE, PUMP 50 g in 100 Part 3 1 TUBE 15 g in 100 Part 4 1 BOTTLE, PLASTIC 15 g in 100 Part 1 of 4 TULA SKINCARE DETOX IN A JAR EXFOLIATING TREATMENT MASK
paste masks (mud packs) pasteProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR LACTIC ACID (UNII: 33X04XA5AT) INGR BLUEBERRY (UNII: 253RUG1X1A) INGR SAFFLOWER OIL (UNII: 65UEH262IS) INGR ASCORBYL PALMITATE (UNII: QN83US2B0N) INGR CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) INGR DECYLENE GLYCOL (UNII: S57M60MI88) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR OCTYLDODECANOL (UNII: 461N1O614Y) INGR TRISODIUM DICARBOXYMETHYL ALANINATE (UNII: 784K2O81WY) INGR PENTYLENE GLYCOL (UNII: 50C1307PZG) INGR ORANGE OIL (UNII: AKN3KSD11B) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR SORBIC ACID (UNII: X045WJ989B) INGR SODIUM GLYCOLATE (UNII: B75E535IMI) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) INGR WATER (UNII: 059QF0KO0R) INGR CHICORY ROOT (UNII: 090CTY533N) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR TITANIUM DIOXIDE (UNII: 15FIX9V2JP) INGR HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR CAMELINA SATIVA SEED OIL (UNII: 12824X01L0) INGR BULNESIA SARMIENTOI WOOD OIL (UNII: 81H0L6W02F) INGR CANANGA OIL (UNII: 8YOY78GNNX) INGR LEMON OIL (UNII: I9GRO824LL) INGR ULTRAMARINE BLUE (UNII: I39WR998BI) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR BENTONITE (UNII: A3N5ZCN45C) INGR KAOLIN (UNII: 24H4NWX5CO) INGR OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR OLIVE OIL (UNII: 6UYK2W1W1E) INGR INULIN (UNII: JOS53KRJ01) INGR LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) INGR XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 2 of 4 TULA SKINCARE KEEP IT CLEAR ACNE FOAM CLEANSER
acne foam cleanser liquidProduct Information Item Code (Source) NDC:72296-909 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength PROPANEDIOL (UNII: 5965N8W85T) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) LACTOCOCCUS LACTIS (UNII: F1A0PSN10V) NIACINAMIDE (UNII: 25X51I8RD4) ALOE VERA LEAF (UNII: ZY81Z83H0X) AZELAIC ACID (UNII: F2VW3D43YT) SODIUM CHLORIDE (UNII: 451W47IQ8X) GLYCERIN (UNII: PDC6A3C0OX) EPILOBIUM ANGUSTIFOLIUM WHOLE (UNII: C278QS9YBT) SACCHARIDE ISOMERATE (UNII: W8K377W98I) XANTHAN GUM (UNII: TTV12P4NEE) BLUEBERRY (UNII: 253RUG1X1A) WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 20 (UNII: 7T1F30V5YH) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) CITRULLUS LANATUS WHOLE (UNII: 3J5I6254YO) CITRUS AURANTIUM FLOWER (UNII: O730ZX2Z83) ORYZA SATIVA WHOLE (UNII: 84IVV0906Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) CETEARYL OLIVATE (UNII: 58B69Q84JO) LACTIC ACID (UNII: 33X04XA5AT) SODIUM LACTATE (UNII: TU7HW0W0QT) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) SORBITAN OLIVATE (UNII: MDL271E3GR) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 Part 3 of 4 TULA BREAKOUT STAR OIL-FREE ACNE MOISTURIZER
acne moisturizer liquidProduct Information Item Code (Source) NDC:72296-908 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength PROPANEDIOL (UNII: 5965N8W85T) AZELAIC ACID (UNII: F2VW3D43YT) CITRULLUS LANATUS WHOLE (UNII: 3J5I6254YO) SACCHARIDE ISOMERATE (UNII: W8K377W98I) NIACINAMIDE (UNII: 25X51I8RD4) CITRUS AURANTIUM FLOWER (UNII: O730ZX2Z83) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) LENTIL (UNII: 6O38V6B52O) EPILOBIUM ANGUSTIFOLIUM WHOLE (UNII: C278QS9YBT) XANTHAN GUM (UNII: TTV12P4NEE) ALOE VERA LEAF (UNII: ZY81Z83H0X) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) LACTOCOCCUS LACTIS (UNII: F1A0PSN10V) POLYSORBATE 20 (UNII: 7T1F30V5YH) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) CETEARYL OLIVATE (UNII: 58B69Q84JO) LACTIC ACID (UNII: 33X04XA5AT) ORYZA SATIVA WHOLE (UNII: 84IVV0906Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) SORBITAN OLIVATE (UNII: MDL271E3GR) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) APPLE (UNII: B423VGH5S9) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 Part 4 of 4 TULA ACNE CLEARING AND TONE CORRECTING GEL
acne correcting gel liquidProduct Information Item Code (Source) NDC:72296-907 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) ZINC (UNII: J41CSQ7QDS) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) EPILOBIUM ANGUSTIFOLIUM WHOLE (UNII: C278QS9YBT) PHENOXYETHANOL (UNII: HIE492ZZ3T) SACCHARIDE ISOMERATE (UNII: W8K377W98I) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM METABISULFITE (UNII: 4VON5FNS3C) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) LACTOCOCCUS LACTIS (UNII: F1A0PSN10V) NIACINAMIDE (UNII: 25X51I8RD4) AZELAIC ACID (UNII: F2VW3D43YT) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 15 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 02/14/2023 Labeler - TULA Life LLC (080051358) Registrant - TULA Life LLC (080051358)