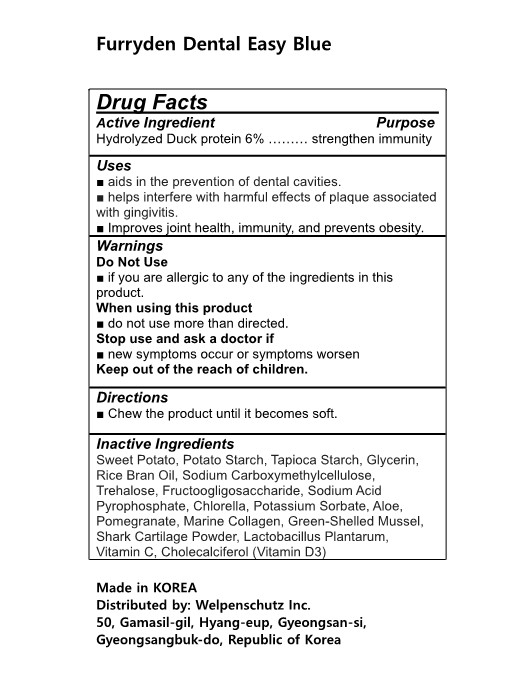
Label: FURRYDEN DENTAL EASY BLUE- hydrolyzed duck protein gum, chewing
- NDC Code(s): 83332-501-01
- Packager: Welpenschutz Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purposes
- Uses
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
- Directions
-
Inactive Ingredients
Sweet Potato, Potato Starch, Tapioca Starch, Glycerin, Rice Bran Oil, Sodium Carboxymethylcellulose, Trehalose, Fructoogligosaccharide, Sodium Acid Pyrophosphate, Chlorella, Potassium Sorbate, Aloe, Pomegranate, Marine Collagen, Green-Shelled Mussel, Shark Cartilage Powder, Lactobacillus Plantarum,
Vitamin C, Cholecalciferol (Vitamin D3)
- Label
-
INGREDIENTS AND APPEARANCE
FURRYDEN DENTAL EASY BLUE
hydrolyzed duck protein gum, chewingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83332-501 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DUCK (UNII: 28ON068RWM) (DUCK - UNII:28ON068RWM) DUCK 6 g in 100 mL Inactive Ingredients Ingredient Name Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) HYDROLYSED MARINE COLLAGEN (ENZYMATIC; 2000 MW) (UNII: 2WID9OCG7P) STARCH, POTATO (UNII: 8I089SAH3T) GLYCERIN (UNII: PDC6A3C0OX) RICE BRAN OIL (UNII: LZO6K1506A) SODIUM ACID PYROPHOSPHATE (UNII: H5WVD9LZUD) STARCH, TAPIOCA (UNII: 24SC3U704I) TREHALOSE (UNII: B8WCK70T7I) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LACTIPLANTIBACILLUS PLANTARUM (UNII: QFC21096ON) CHOLECALCIFEROL (UNII: 1C6V77QF41) ALOE (UNII: V5VD430YW9) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SHARK CARTILAGE (UNII: D2YCN1I522) SWEET POTATO (UNII: M9WGG9Z9GK) NEW ZEALAND GREEN MUSSEL (UNII: 1L5332YQ7U) FRUCTOSE (UNII: 6YSS42VSEV) CHLORELLA VULGARIS (UNII: RYQ4R60M02) Product Characteristics Color blue Score score with uneven pieces Shape ROUND Size 5mm Flavor BLUEBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83332-501-01 200 mL in 1 POUCH; Type 0: Not a Combination Product 03/15/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/17/2023 Labeler - Welpenschutz Inc. (695930167) Registrant - Welpenschutz Inc. (695930167) Establishment Name Address ID/FEI Business Operations Welpenschutz Inc. 695930167 manufacture(83332-501)