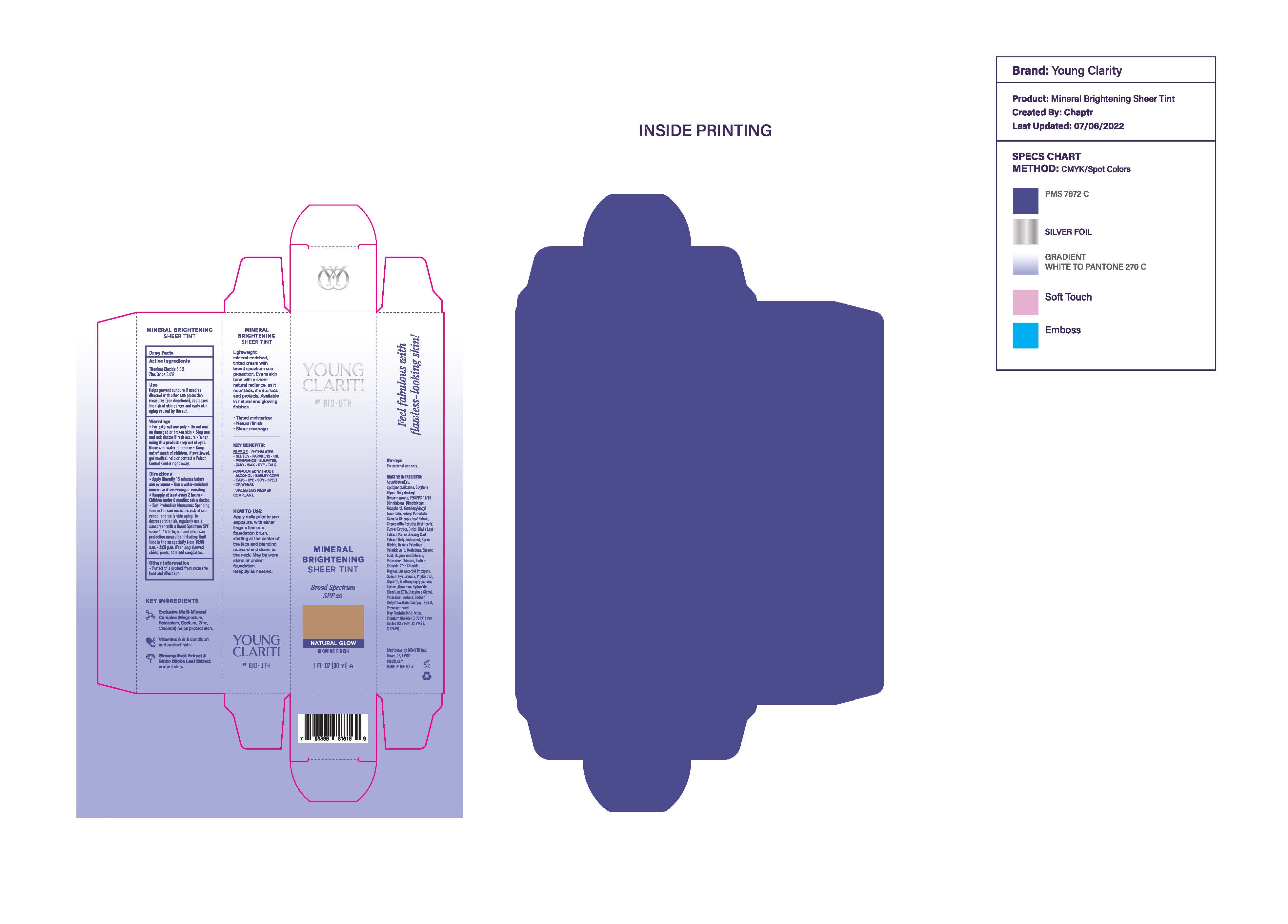
Label: YOUNG CLARITI MINERAL BRIGHTENING SHEER TINT BROADSPECTRUM SPF 20- brightening sheer tint cream
- NDC Code(s): 82964-851-01
- Packager: BioYouth Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Use
- Warnings
-
Directions
Apply liberally 15 minutes before sun exposure
Reapply at least every 2 hours
Use water resistant sunscreen if swimming or sweating
Sun Protection Measures: Spending time in the sun increases risk of skin cancer and early aging. To decrease this risk, regular use of a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun especially from 10:00 am - 2:00 pm. Wear long sleeved shirts, pants, hats and sunglasses.
Children under 6 months: ask a doctor -
Inactive Ingredients
Aqua/Water /Eau, Cyclopentasiloxane,Butylene Glycol, Octyldodecyl Neopentanoate, PEG/PPG-18/18 Dimethicone, Dimethicone,
Tocopherol, Tetrahexyldecyl Ascorbate,Retinyl Palmitate,Camellia Sinensis Leaf Extract, Chamomilla Recutita (Matricaria) Flower Extract, Ginko Biloba Leaf Extract, Panax Ginsing Root Extract, Octyldodecanol, Boron Nitride, Dextrin Palmitate, Palmitic Acid, Methicone, Stearic Acid, Magnesium Chloride, Potassium Chloride, Sodium Chloride, Zinc Chloride,Magnesium Ascorbyl Phosphate, Sodium Hyaluronate, Phytantriol, Glycerin, Triethoxycaprylylsilane,Lysine, Aluminum Hydroxide,Disodium EDTA,Hexylene Glycol, Potassium Sorbate, Sodium Dehydroacetate, Caprylyl Glycol, Phenoxyethanol. May Contain (+/-): Mica, Titanium (CI 77891), Iron Oxides (CI 77491,CI 77492, CI 77499).
- Other Information
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- Pinciple Display Panel
-
INGREDIENTS AND APPEARANCE
YOUNG CLARITI MINERAL BRIGHTENING SHEER TINT BROADSPECTRUM SPF 20
brightening sheer tint creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82964-851 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 5 g in 30 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 5 g in 30 mL Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) PALMITIC ACID (UNII: 2V16EO95H1) OCTYLDODECANOL (UNII: 461N1O614Y) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) PHYTANTRIOL (UNII: 8LVI07A72W) DEXTRIN PALMITATE (CORN; 20000 MW) (UNII: 89B2BSF9I3) HEXYLENE GLYCOL (UNII: KEH0A3F75J) TOCOPHEROL (UNII: R0ZB2556P8) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) FERRIC OXIDE RED (UNII: 1K09F3G675) ZINC CHLORIDE (UNII: 86Q357L16B) ASIAN GINSENG (UNII: CUQ3A77YXI) METHICONE (20 CST) (UNII: 6777U11MKT) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MICA (UNII: V8A1AW0880) WATER (UNII: 059QF0KO0R) GINKGO (UNII: 19FUJ2C58T) DIMETHICONE (UNII: 92RU3N3Y1O) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYALURONIC ACID (NON-ANIMAL STABILIZED) (UNII: B7SG5YV2SI) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) LYSINE (UNII: K3Z4F929H6) STEARIC ACID (UNII: 4ELV7Z65AP) BORON NITRIDE (UNII: 2U4T60A6YD) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CHAMOMILE (UNII: FGL3685T2X) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Product Characteristics Color brown (NATURAL GLOW) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82964-851-01 1 in 1 CARTON 02/28/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/28/2023 Labeler - BioYouth Inc. (118700923) Registrant - MANA Products, Inc (078870292) Establishment Name Address ID/FEI Business Operations MANA Products, Inc 032870270 manufacture(82964-851) Establishment Name Address ID/FEI Business Operations MANA Products, Inc. 078870292 manufacture(82964-851)


 Protect this product from excessive heat and direct sun
Protect this product from excessive heat and direct sun
