Label: MINIVLAR 30- ethinyl estradiol, levonorgestrel tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 72689-0011-1 - Packager: OASIS TRADING
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 21, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
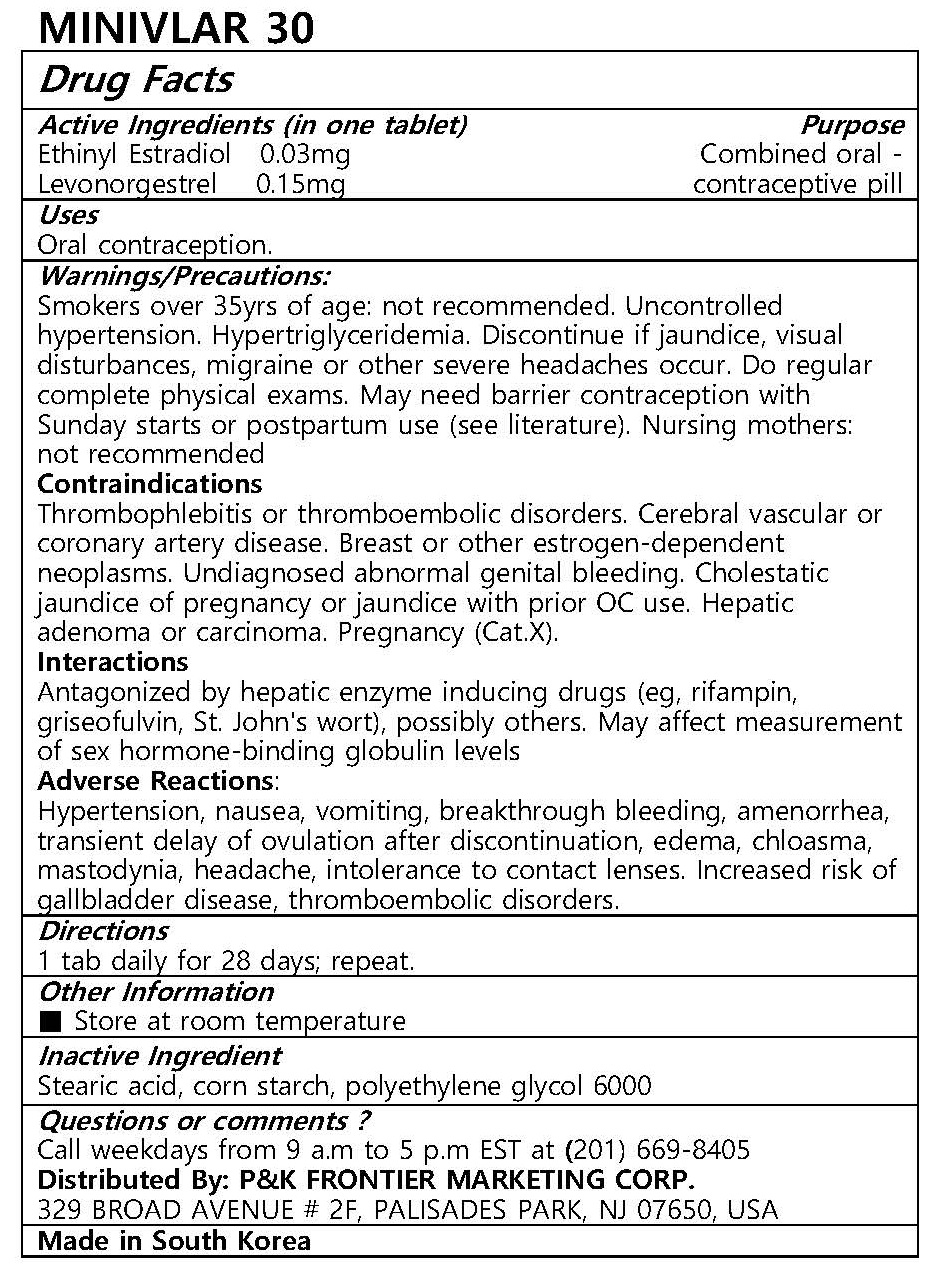
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings/Precautions:
Smokers over 35yrs of age: not recommended. Uncontrolled hypertension. Hypertriglyceridemia. Discontinue if jaundice, visual disturbances, migraine or other severe headaches occur. Do regular complete physical exams. May need barrier contraception with Sunday starts or postpartum use (see literature). Nursing mothers: not recommended
Contraindications
Thrombophlebitis or thromboembolic disorders. Cerebral vascular or coronary artery disease. Breast or other estrogen-dependent neoplasms. Undiagnosed abnormal genital bleeding. Cholestatic jaundice of pregnancy or jaundice with prior OC use. Hepatic adenoma or carcinoma. Pregnancy (Cat.X).
Interactions
Antagonized by hepatic enzyme inducing drugs (eg, rifampin, griseofulvin, St. John's wort), possibly others. May affect measurement of sex hormone-binding globulin levels
Adverse Reactions:
Hypertension, nausea, vomiting, breakthrough bleeding, amenorrhea, transient delay of ovulation after discontinuation, edema, chloasma, mastodynia, headache, intolerance to contact lenses. Increased risk of gallbladder disease, thromboembolic disorders. - INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINIVLAR 30
ethinyl estradiol, levonorgestrel tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72689-0011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETHINYL ESTRADIOL (UNII: 423D2T571U) (ETHINYL ESTRADIOL - UNII:423D2T571U) ETHINYL ESTRADIOL 0.03 mg LEVONORGESTREL (UNII: 5W7SIA7YZW) (LEVONORGESTREL - UNII:5W7SIA7YZW) LEVONORGESTREL 0.15 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) Product Characteristics Color white Score no score Shape ROUND Size 5mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72689-0011-1 21 in 1 BLISTER PACK; Type 0: Not a Combination Product 11/21/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/21/2018 Labeler - OASIS TRADING (689991468) Registrant - OASIS TRADING (689991468) Establishment Name Address ID/FEI Business Operations OASIS TRADING 689991468 manufacture(72689-0011) , label(72689-0011)