Label: ALFAXAN MULTIDOSE- alfaxalone injection, solution
- NDC Code(s): 54771-6696-1, 54771-6696-2
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated May 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
ALFAXAN MULTIDOSE (alfaxalone) is a neuroactive steroid molecule with properties of a general anesthetic. Alfaxalone is chemically described as 3-α-hydroxy-5-α-pregnane-11, 20-dione, and has a molecular weight 332.5. The primary mechanism for the anesthetic action of alfaxalone is modulation of neuronal cell membrane chloride ion transport, induced by binding of alfaxalone to GABAA (gamma-aminobutyric acid) cell surface receptors.
- INDICATIONS
-
DOSAGE AND ADMINISTRATION
Administer by intravenous injection only. For induction, administer ALFAXAN MULTIDOSE over approximately 60 seconds or until clinical signs show the onset of anesthesia, titrating administration against the response of the patient. Rapid administration of ALFAXAN MULTIDOSE may be associated with an increased incidence of cardiorespiratory depression or apnea. Apnea can occur following induction or after the administration of maintenance boluses of ALFAXAN MULTIDOSE. The use of preanesthetics may reduce the ALFAXAN MULTIDOSE induction dose. The choice and the amount of phenothiazine, alpha2-adrenoreceptor agonist, benzodiazepine or opioid will influence the response of the patient to an induction dose of ALFAXAN MULTIDOSE.
When using ALFAXAN MULTIDOSE, patients should be continuously monitored, and facilities for the maintenance of a patent airway, artificial ventilation, and oxygen supplementation must be immediately available.
ALFAXAN MULTIDOSE contains preservatives. Use within 56 days of first puncture. Any unused ALFAXAN MULTIDOSE remaining after 56 days should be discarded.
ALFAXAN MULTIDOSE should not be mixed with other therapeutic agents prior to administration.
-
CATS
Induction of general anesthesia in cats: Induction dose guidelines are based on data from the field study (see EFFECTIVENESS) and range between 2.2 - 9.7 mg/kg for cats that did not receive a preanesthetic, and between 1.0 - 10.8 mg/kg for cats that received a preanesthetic. The alfaxalone induction dose in the field study was reduced by 10 - 43%, depending on the combination of preanesthetics (dose sparing effect). Dose sparing of ALFAXAN MULTIDOSE will depend on the potency, dose, and time of administration of the various preanesthetics that are used prior to induction. To avoid anesthetic overdose, titrate the administration of ALFAXAN MULTIDOSE against the response of the patient.
Anesthesia is usually observed within 60 seconds after the start of injection, and permits intubation within 1 - 2 minutes, irrespective of preanesthetic. The duration of anesthesia from a single induction dose ranges between 15 - 30 minutes in the unpreanesthetized cat. If a preanesthetic is used, anesthetic duration may be longer, depending on the class and dose of preanesthetic. Individual anesthesia times vary.
Examples from the field study of average induction doses (and ranges) for cats that received various preanesthetics are presented as dosing guidelines in the table. The table is for guidance only. Draw up the maximum expected target dose and administer to effect. The actual induction dose should be based on patient response.
Alfaxalone Induction Dose Guidelines: CATS Preanesthetic Average alfaxalone induction dose (and range) in mg/kg Number of cats No preanesthetic 4.0 (2.2-9.7) 33 Opioid + phenothiazine 3.2 (1.1-10.8) 96 Benzodiazepine + phenothiazine 3.6 (1.5-7.1) 23 Benzodiazepine + opioid + phenothiazine 2.3 (1.2-5.0) 26 Alpha2-adrenergic agonist with/without phenothiazine 3.6 (1.1-5.0) 15 Alpha2-adrenergic agonist + phenothiazine with/without benzodiazepine or opioid 2.9 (1.0-3.9) 11 Additional doses of ALFAXAN MULTIDOSE similar to those used for maintenance (1.1 - 1.5 mg/kg) may be administered to facilitate intubation.
Maintenance of general anesthesia in cats: Following induction of anesthesia with ALFAXAN MULTIDOSE and intubation, anesthesia may be maintained using intermittent ALFAXAN MULTIDOSE intravenous boluses or an inhalant anesthetic agent. A maintenance bolus containing 1.1 - 1.3 mg/kg provides an additional 7 - 8 minutes of anesthesia in preanesthetized cats. A dose of 1.4 - 1.5 mg/kg provides an additional 3 - 5 minutes anesthesia in unpreanesthetized cats. Clinical response may vary, and is determined by the dose, rate of administration, and frequency of maintenance injections.
ALFAXAN MULTIDOSE maintenance dose sparing is greater in cats that receive a preanesthetic. Examples from the field study of maintenance doses for preanesthetized and unpreanesthetized cats are presented as guidelines in the table. Maintenance dose and frequency should be based on the response of the individual patient.
Alfaxalone Maintenance Dose Guidelines: CATS Dose and Duration Preanesthetized Cats Unpreanesthetized Cats Maintenance anesthesia doses 1.1 - 1.3 mg/kg 1.4 - 1.5 mg/kg Mean duration of anesthesia 7-8 minutes 3 - 5 minutes In the field study, recovery times (extubation to head lift) following alfaxalone maintenance anesthesia averaged 15 minutes in cats that did not receive a preanesthetic, and 17 minutes in preanesthetized cats.
Inhalant anesthetic maintenance of general anesthesia in cats: Additional low doses of ALFAXAN MULTIDOSE, similar to a maintenance dose, may be required to facilitate the transition to inhalant maintenance anesthesia.
-
DOGS
Induction of general anesthesia in dogs: Induction dose guidelines are based on data from the field study (see EFFECTIVENESS) and range between 1.5 - 4.5 mg/kg for dogs that did not receive a preanesthetic, and between 0.2 - 3.5 mg/kg for dogs that received a preanesthetic. The alfaxalone induction dose in the field study was reduced by 23 - 50% depending on the combination of preanesthetics (dose sparing effect). Dose sparing of ALFAXAN MULTIDOSE will depend on the potency, dose, and time of administration of the various preanesthetics that are used prior to induction. To avoid anesthetic overdose, titrate the administration of ALFAXAN MULTIDOSE against the response of the patient. In the field study, the use of a preanesthetic appeared to decrease the occurrence of apnea following alfaxalone induction in dogs.
In dogs, ALFAXAN MULTIDOSE usually produces recumbence within 60 seconds after the start of injection, and permits intubation within 1 - 2 minutes, irrespective of preanesthetic. The duration of anesthesia from a single induction dose is approximately 5 - 10 minutes in the unpreanesthetized dog. If a preanesthetic is used, anesthetic duration may be longer, depending on the class and dose of preanesthetic. Individual anesthesia times vary.
Examples from the field study of average induction doses (and ranges) for dogs that received various preanesthetics are presented as dosing guidelines in the table. The table is for guidance only. Draw up the maximum expected target dose and administer to effect. The actual induction dose should be based on patient response.
Alfaxalone Induction Dose Guidelines: DOGS Preanesthetic Average alfaxalone induction dose (and range) in mg/kg Number of dogs No preanesthetic 2.2 (1.5 - 4.5) 17 Benzodiazepine + opioid + acepromazine 1.7 (0.9 - 3.5) 39 Opioid + acepromazine 1.6 (0.6 - 3.5) 80 Alpha2-agonist 1.1 (0.21 - 2.00) 9 Additional doses of ALFAXAN MULTIDOSE similar to those used for maintenance (1.2 - 2.2 mg/kg) may be administered to facilitate intubation.
Maintenance of general anesthesia in dogs: Following induction of anesthesia with ALFAXAN MULTIDOSE and intubation, anesthesia may be maintained using intermittent ALFAXAN MULTIDOSE intravenous boluses or an inhalant anesthetic agent. A maintenance bolus containing 1.2 - 1.4 mg/kg provides an additional 6 - 8 minutes anesthesia in preanesthetized dogs. A dose of 1.5 - 2.2 mg/kg provides an additional 6 - 8 minutes of anesthesia in unpreanesthetized dogs. Clinical response may vary, and is determined by the dose, rate of administration, and frequency of maintenance injections.
ALFAXAN MULTIDOSE maintenance dose sparing is greater in dogs that receive a preanesthetic. Examples from the field study of maintenance doses for preanesthetized and unpreanesthetized dogs are presented as guidelines in the table. Maintenance dose and frequency should be based on the response of the individual patient.
Alfaxalone Maintenance Dose Guidelines: DOGS Dose and Duration Preanesthetized Dogs Unpreanesthetized Dogs Maintenance anesthesia doses 1.2 - 1.4 mg/kg 1.5 - 2.2 mg/kg Mean duration of anesthesia 6 - 8 minutes 6 - 8 minutes In the field study, recovery times (extubation to head lift) following alfaxalone maintenance anesthesia averaged 22 minutes in dogs that did not receive a preanesthetic, and 15 minutes in preanesthetized dogs.
Inhalant anesthetic maintenance of general anesthesia in dogs: Additional low doses of ALFAXAN MULTIDOSE, similar to a maintenance dose, may be required to facilitate the transition to inhalant maintenance anesthesia.
-
DRUG INTERACTIONS
No specific preanesthetic is either indicated or contraindicated with ALFAXAN MULTIDOSE. The necessity for and choice of preanesthetic is left to the discretion of the veterinarian. Preanesthetic doses may be lower than the label directions for their use as a single medication. ALFAXAN MULTIDOSE is compatible with benzodiazepines, opioids, alpha2-agonists, and phenothiazines as commonly used in surgical practice.
In the field study, alfaxalone was used safely in cats and dogs that received frequently used veterinary products, including antibiotics, anticholinergics, vaccines, steroids, and dewormers.
- CONTRAINDICATIONS
-
WARNINGS
Animal safety: When using ALFAXAN MULTIDOSE, patients should be continuously monitored, and facilities for the maintenance of a patent airway, artificial ventilation, and oxygen supplementation must be immediately available.
Rapid bolus administration or anesthetic overdose may cause cardiorespiratory depression, including hypotension, apnea, hypoxia, or death. Arrhythmias may occur secondary to apnea and hypoxia. In cases of anesthetic overdose, stop ALFAXAN MULTIDOSE administration and administer treatment as indicated by the patient’s clinical signs. Cardiovascular depression should be treated with plasma expanders, pressor agents, anti-arrhythmic agents or other techniques as appropriate for the treatments of the clinical signs.
Human safety: Not for human use. Keep out of the reach of children.
ALFAXAN MULTIDOSE should be managed to prevent the risk of diversion, through such measures as restriction of access and the use of drug accountability procedures appropriate to the clinical setting.
Exercise caution to avoid accidental self-injection. Overdose is likely to cause cardiorespiratory depression (such as hypotension, bradycardia and/or apnea). Remove the individual from the source of exposure and seek medical attention. Respiratory depression should be treated by artificial ventilation and oxygen.
Avoid contact of this product with skin, eyes, and clothes. In case of contact, eyes and skin should be liberally flushed with water for 15 minutes. Consult a physician if irritation persists. In the case of accidental human ingestion, seek medical advice immediately and show the package insert or the label to the physician.
Note to physician: This product contains an injectable anesthetic. - CONTACT INFORMATION
-
DRUG ABUSE AND DEPENDENCE
Controlled substance: ALFAXAN MULTIDOSE contains alfaxalone a neurosteroid anesthetic and a class IV controlled substance.
Abuse: Alfaxalone is a central nervous system depressant that acts on GABA receptor associated chloride channels, similar to the mechanism of action of Schedule IV sedatives such as benzodiazepines (diazepam and midazolam), barbiturates (phenobarbital and methohexital) and fospropofol. In a drug discrimination behavioral test in rats, the effects of alfaxalone were recognized as similar to those of midazolam. These biochemical and behavioral data suggest that alfaxalone has an abuse potential similar to other Schedule IV sedatives.
Physical dependence: There are no data that assess the ability of alfaxalone to induce physical dependence. However, alfaxalone has a mechanism of action similar to the benzodiazepines and can block the behavioral responses associated with precipitated benzodiazepine withdrawal. Therefore, it is likely that alfaxalone can also produce physical dependence and withdrawal signs similar to that produced by the benzodiazepines.
Psychological dependence: The ability of alfaxalone to produce psychological dependence is unknown because there are no data on the rewarding properties of the drug from animal self-administration studies or from human abuse potential studies. -
PRECAUTIONS
Rapid arousal: Careful monitoring of the patient is necessary due to possibility of rapid arousal.
Preanesthesia: Benzodiazepines may be used safely prior to ALFAXAN MULTIDOSE in the presence of other preanesthetics (see DRUG INTERACTIONS). However, when a benzodiazepine was used as the sole preanesthetic, excitation occurred in some cats and dogs during ALFAXAN MULTIDOSE anesthesia and recovery.
Apnea: Apnea may occur following administration of an induction dose, a maintenance dose or a dose administered during the transition to inhalant maintenance anesthesia, especially with higher doses and rapid administration. Endotracheal intubation, oxygen supplementation, and intermittent positive pressure ventilation (IPPV) should be administered to treat apnea and associated hypoxemia.
Blood Pressure: The myocardial depressive effects of ALFAXAN MULTIDOSE combined with the vasodilatory effects of inhalant anesthetics can be additive, resulting in hypotension. Preanesthetics may increase the anesthesia effect of ALFAXAN MULTIDOSE and result in more pronounced changes in systolic, diastolic, and mean arterial blood pressures. Transient hypertension may occur, possibly due to elevated sympathetic activity.
Body Temperature: A decrease in body temperature occurs during ALFAXAN MULTIDOSE anesthesia unless an external heat source is provided. Supplemental heat should be provided to maintain acceptable core body temperature until full recovery.
Breeding Animals: ALFAXAN MULTIDOSE has not been evaluated in pregnant, lactating, and breeding cats. Alfaxalone crosses the placenta, and as with other general anesthetic agents, the administration of ALFAXAN MULTIDOSE may be associated with neonatal depression.
Kittens and Puppies: ALFAXAN MULTIDOSE has not been evaluated in cats less than 4 weeks of age or in dogs less than 10 weeks of age.
Compromised or Debilitated Cats and Dogs: The administration of ALFAXAN MULTIDOSE to debilitated patients or patients with renal disease, hepatic disease, or cardiorespiratory disease has not been evaluated. Doses may need adjustment for geriatric or debilitated patients. Caution should be used in cats or dogs with cardiac, respiratory, renal or hepatic impairment, or in hypovolemic or debilitated cats and dogs, and geriatric animals.
Analgesia during anesthesia: Appropriate analgesia should be provided for painful procedures.
-
ADVERSE REACTIONS
Adverse Reactions in Cat Field Study Adverse Reaction Number of Catsa = 207 Hypotension (≤90 mm Hg) 92 Tachycardia (≥ 180 bpm) 61 Apnea (≥ 30 seconds) 32 (of 202) Hypertension (> 165 mm Hg) 23 Bradypnea (RR< 10 breaths/min) 16 Apnea (≥ 60 seconds) 12 (of 202) Bradycardia (≤90 beats/min) 10 Hypothermia (<97°F) 10 Hypoxia (SpO2 <85%) 4 Emesis 1 Unacceptable Anesthesia Quality 1 aEach cat may have experienced more than one adverse reaction Additional adverse reactions for cats included vocalization, paddling, and muscle tremors. One cat that experienced tachycardia and hypoxia during anesthesia was euthanized 3 days later due to carcinoma involving the liver, pancreas and common bile duct. The relationship of the original tachycardia during anesthesia and the carcinoma is unknown.
Adverse Reactions in Dog Field Study Adverse Reaction Number of Dogsa = 182 Bradypnea (RR< 10 breaths/min) 89 Apnea (≥ 30 seconds) 55 (of 137) Hypertension (> 165 mm Hg) 54 Tachycardia (≥ 180 bpm) 49 Apnea (≥ 60 seconds) 34 (of 137) Hypotension (≤70 mm Hg) 32 Hypothermia (<97°F) 28 Bradycardia (≤70 beats/min) 24 Hypoxia (SpO2 <85%) 4 Lack of Effectiveness 3 Unacceptable Anesthesia Quality 1 Emesis 1 aEach dog may have experienced more than one adverse reaction Additional adverse reactions for dogs included vocalization, paddling, and muscle tremors.
To report adverse reactions or to obtain a copy of the SDS for this product call 1-888-963-8471.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
-
OVERDOSE
Rapid administration, accidental overdose, or relative overdose due to inadequate dose sparing of ALFAXAN MULTIDOSE in the presence of preanesthetics may cause cardiopulmonary depression. Respiratory arrest (apnea) may be observed. In cases of respiratory depression, stop drug administration, establish a patent airway, and initiate assisted or controlled ventilation with pure oxygen. Cardiovascular depression should be treated with plasma expanders, pressor agents, antiarrhythmic agents or other techniques as appropriate for the observed abnormality.
-
CLINICAL PHARMACOLOGY
ALFAXAN MULTIDOSE is a reformulation of previously approved, single-use ALFAXAN (NADA 141-342) which contained no antimicrobial preservative. The bioequivalence of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE following administration of 5 mg/kg IV was demonstrated in a two sequence, two period crossover study in 24 cats (see EFFECTIVENESS). The bioequivalence of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE following administration of 3 mg/kg IV was demonstrated in a two sequence, two period crossover study in 24 dogs (see EFFECTIVENESS).
-
EFFECTIVENESS
Cat bioequivalence study: The bioequivalence of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE following administration of 5 mg/kg IV was demonstrated in a two sequence, two period crossover study in 24 cats. The two products were bioequivalent because the upper and lower bounds of the 90% confidence intervals for Cmax and AUClast of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE met the bioequivalence criteria of 80 - 125%.
Table 1. Summary of bioequivalence parameters following a single IV administration of 5 mg/kg in cats of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE: Parameter Product Geo LS Mean1 T/R* Lower Bound2 Upper Bound2 Cmax (g/mL) ALFAXAN (unpreserved) 8.45 0.97 91.58% 102.38% ALFAXAN MULTIDOSE 8.18 AUClast (min*g/mL) ALFAXAN (unpreserved) 186.07 0.98 93.11% 102.77% ALFAXAN MULTIDOSE 182.01 *T/R = Test/Reference = ALFAXAN MULTIDOSE/ALFAXAN (unpreserved)
1Geometric means
2Lower and upper 90% confidence bounds for the ratio of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE
Dog bioequivalence study: The bioequivalence of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE following administration of 3 mg/kg IV was demonstrated in a two sequence, two period crossover study in 24 dogs. The two products were bioequivalent, because the upper and lower bounds of the 90% confidence intervals for Cmax and AUClast of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE met the bioequivalence criteria of 80 - 125%.
Table 2. Summary of bioequivalence parameters following a single IV administration of 3 mg/kg in dogs of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE: Parameter Product Geo LS Mean1 T/R* Lower Bound2 Upper Bound2 Cmax (g/mL) ALFAXAN (unpreserved) 4.42 1.07 100.26% 114.57% ALFAXAN MULTIDOSE 4.74 AUClast (min*g/mL) ALFAXAN (unpreserved) 74.77 1.06 101.52% 109.72% ALFAXAN MULTIDOSE 78.92 *T/R = Test/Reference = ALFAXAN MULTIDOSE/ALFAXAN (unpreserved)
1Geometric means
2Lower and upper 90% confidence bounds for the ratio of ALFAXAN (unpreserved) and ALFAXAN MULTIDOSE
Cat Field Study: Two hundred and seven cats of 19 breeds, between the ages of 1 month to 17 years, weighing between 0.6-9 kg, were successfully anesthetized with alfaxalone (unpreserved) for various types of surgery or procedures requiring anesthesia. Induction doses ranged between 1.0-10.8 mg/kg for cats that received preanesthetics, and between 2.2-9.7 mg/kg for unpreanesthetized cats (see DOSAGE AND ADMINISTRATION for doses by preanesthetic treatment groups). For most cats, the alfaxalone induction dose was reduced (10-43%), depending on the combination of preanesthetics (dose sparing effect). One hundred and four cats were maintained using an inhalant anesthetic; 72 cats were maintained using between 1 to 5 alfaxalone boluses. Mean alfaxalone maintenance doses ranged between 1.1-1.3 mg/kg in preanesthetized cats and 1.4-1.5 in unpreanesthetized cats. Doses were given to effect and titrated against the response of the individual patient.
All cats in the field study were intubated and received supplemental oxygen. Apnea ≥30 seconds occurred in 28 (of 169) preanesthetized cats and 4 (of 33) unpreanesthetized cats after induction with alfaxalone. Apnea continued ≥60 seconds in 9 of the 28 apneic preanesthetized cats and 3 of the 4 apneic unpreanesthetized cats after induction with alfaxalone. Other adverse reactions included hypotension, tachycardia, hypertension, bradypnea, bradycardia, and hypothermia (see ADVERSE REACTIONS).
In the field study, recovery times (extubation to head lift) following alfaxalone maintenance anesthesia averaged 15 minutes in cats that did not receive a preanesthetic, and 17 minutes in preanesthetized cats. Average recovery times following the use of an inhalant anesthetic ranged between 1-95 minutes (mean 14 minutes).
Dog field study: One hundred eighty-two dogs of 54 breeds, between the ages of 3 months to 13 years, weighing between 2.4 and 41 kg, were successfully anesthetized with alfaxalone (unpreserved) for various types of surgery or procedures requiring anesthesia. Induction doses ranged between 0.2 - 3.5 mg/kg for preanesthetized dogs, and between 1.5 - 4.5 mg/kg for dogs that did not receive a preanesthetic (see DOSAGE AND ADMINISTRATION for doses by preanesthetic treatment groups). The alfaxalone induction dose in the field study was reduced by 23-50% depending on the combination of preanesthetics (dose sparing effect). One hundred and eighteen dogs were maintained using an inhalant anesthetic; 17 dogs were maintained using between 1-5 alfaxalone boluses. Alfaxalone maintenance doses ranged between 1.2 - 1.4 mg/kg in preanesthetized dogs and 1.5 - 2.2 in unpreanesthetized dogs. Doses were given to effect and titrated against the response of the individual patient.
All dogs in the field study were intubated and received supplemental oxygen. Following induction using alfaxalone, apnea ≥30 seconds occurred in 46 (of 123) preanesthetized dogs and 9 (of 17) unpreanesthetized dogs. Apnea continued for ≥60 seconds in 18 of the 46 apneic preanesthetized dogs and 8 of the 9 apneic unpreanesthetized dogs after induction with alfaxalone. The duration of apnea ranged between 38 seconds and 6 minutes, 47 seconds. Of the 17 dogs that received up to 5 alfaxalone maintenance boluses, 11 (64.7%) experienced 14 periods of apnea, averaging 2.6 minutes each. Other adverse reactions included bradypnea, hypotension, tachycardia, hypertension, hypothermia, and bradycardia (see ADVERSE REACTIONS).
-
ANIMAL SAFETY
Plasma concentrations of alfaxalone over time after IV administration of ALFAXAN (unpreserved) or ALFAXAN MULTIDOSE to cats and dogs were compared and found to be bioequivalent for AUClast and Cmax (see CLINICAL PHARMACOLOGY). Monitoring of physiological variables, evaluation of anesthetic induction, anesthetic effectiveness, anesthetic recovery, and anesthetic event times during the bioequivalence study showed that the two formulations result in similar pharmacodynamic effects. The demonstrated blood level bioequivalence supports the systemic safety of the ALFAXAN MULTIDOSE formulation.
Cat multiple dose safety study: In a multiple dose safety study, 5 groups of 6 healthy cats (half male, half female) were administered ALFAXAN at 0 (saline), 5, 15 and 25 mg/kg on days 0, 2 and 5, at 48 hour intervals. Variables included induction and recovery times, heart rate (HR), respiratory rate (RR), indirect blood pressure (BP), clinical pathology, and necropsy. Anesthetic and cardiorespiratory variables were collected prior to induction and at 10 minute intervals after each induction until recumbence. Electrocardiograms (ECG) were monitored at observation time points.
Recovery time increased with increasing dose. Increasing doses of alfaxalone resulted in decreases in heart rate, respiratory rate, and blood pressure within 15 minutes postinduction. The lowest RR (18 breaths per minute) seen at 15 and 25 mg/kg occurred at 50 and 5 minutes post-dose respectively. Cats in the 5 mg/kg dose group reached a minimum of 23 breaths per minute at 10 minutes post-dose. During the initial 5 minutes after induction, there was 1 episode of apnea at 5 mg/kg, 6 episodes of apnea at 15 mg/kg, and 3 episodes of apnea at 25 mg/kg. Decreases in mean hemoglobin saturation (SpO2) were not dose related. The lowest mean hemoglobin concentration for cats in both the 5 and 15 mg/kg dose groups were approximately 88%. For cats that received 25 mg/kg, the lowest SpO2 was 83%. Mean systolic and diastolic blood pressure decreased with increasing dose. No abnormal cardiac arrhythmias were noted during the study (ECG observed but not recorded). Clinical pathology abnormalities were not clinically significant for all groups. Abnormal necropsy and histopathology findings were associated with injection site trauma consistent with intravenous injection and repeat catheterization. No pain on injection was reported.
The most common adverse reactions were post-anesthetic coughing, fluid in the endotracheal tube, and increased airway sounds. One death occurred in the 25 mg/kg group due to complications associated with a traumatic fall following extubation.
Cat preanesthetic compatibility study: Thirty healthy cats (15 female and 15 male cats) were allocated to each of 5 preanesthetic treatment groups. Alfaxalone dose sparing and the cardiovascular and respiratory interaction of alfaxalone when administered following intramuscular preanesthetic administration of acepromazine, medetomidine, midazolam, butorphanol, or saline, were evaluated. No procedures were performed; no cat received maintenance anesthesia.
Preanesthetic, preanesthetic dose, alfaxalone dose, and duration of anesthesia Preanesthetic (IM) Preanesthetic Dose Alfaxalone IV Induction Dose (mg/kg) Average Duration of Anesthesia (min) medetomidine 100 mcg/kg 2.2 98.2 acepromazine 1.1 mg/kg 2.7 36.3 butorphanol 0.4 mg/kg 2.8 26.5 0.9% saline 0 mg/kg 3.0 26.1 midazolam 0.1 mg/kg 3.3 16.7 Cats given midazolam as the sole preanesthetic required more alfaxalone than the saline group. Durations of recovery increased with the duration of anesthesia. Physiologic variables (HR, RR, BP, SpO2) remained satisfactory during anesthesia and reflected the effects primarily of the associated preanesthetic. Transient cardiac arrhythmias were noted during alfaxalone anesthesia in several cats. Three cats preanesthetized with medetomidine experienced sinus arrhythmias (1 prior to alfaxalone) and 3 were bradycardic (HR <110 bpm). Two cats that received midazolam preanesthesia showed isolated ventricular premature contractions (VPC; 1 prior to alfaxalone).
The quality of anesthesia based on overall anesthetic scores was acceptable for all groups. However, the quality of midazolam preanesthesia, when used alone prior to alfaxalone anesthesia, was less satisfactory compared with other preanesthetics.
Cat tolerance safety study: Eight adult, healthy cats (4 male and 4 female) received 0 (saline), 5, 15, and 50 mg/kg of alfaxalone over 2 days in a dose escalation design, with at least 3 hours between doses.
Decreases in HR, RR, decreases in PaO2, and increases in PaCO2 were related to dose. All cardiopulmonary variables returned to baseline values by 15 minutes (5 mg/kg), 30 minutes (15 mg/kg) and 1 hour (50 mg/kg) after alfaxalone administration. The 50 mg/kg dose produced marked cardiovascular depression lasting from 10 to 30 minutes. Five of seven cats dosed at 50 mg/kg were euthanised due to prolonged hypoxia after 5 hours of anesthesia.
Apnea occurred at all doses. Respiratory depression and apnea (duration averaging 21 seconds, 63 seconds and 28 minutes) were observed at the 5, 15 and 50 mg/ kg doses, respectively. The duration of apnea generally increased with the alfaxalone dose, occurring more often and for longer duration at 15 and 50 mg/kg. One cat experienced apnea lasting 3 minutes at 5 mg/kg. Tracheal intubation and administration of 100% oxygen and manual artificial ventilation were needed to raise arterial PaO2 from < 60 mm Hg to > 80 mm Hg. Five cats received oxygen at 5 mg/kg, 7 received oxygen at 15 mg/kg, and all cats required oxygen at 50 mg/kg. Other adverse reactions at 5 mg/kg included 1 cat with cyanotic mucous membranes, and 1 cat with fluid in the endotracheal tube.
Duration of anesthesia increased with higher doses, lasting 26, 83, and 126 minutes after administration of 5, 15, and 50 mg/kg, respectively. Average quality scores (1, 2 or 3 - with 1 being the best) for induction and anesthesia were 1 for cats that received the 5 or 15 mg/kg doses. Average quality scores for recovery were 1 and 1.1 for the 5 and 15 mg/kg groups, respectively.
Dog multiple dose safety study: In a multiple dose safety study, 4 groups of 6 healthy Beagle dogs (3 male, 3 female) were administered alfaxalone (unpreserved) at 0 (saline), 2, 6, and 10 mg/kg, 3 times at 48 hour intervals. Variables included induction and recovery times, HR, RR, indirect BP, clinical pathology, urinalysis, and necropsy. Anesthetic and cardiorespiratory variables were collected prior to induction and at 10 minute intervals after each induction until recumbency. Health observations, clinical pathology, and urinalysis variables were collected during the study on non-treatment days.
Induction times decreased and recovery times increased with relation to the anesthetic dose. Body temperature decreased in proportion to the dose and the length of anesthesia. The minimum rectal temperature recorded was 98.3°F. There was a dose related decrease in SpO2, respiratory rate, and blood pressures. Mean heart rates increased with the increase in alfaxalone dosage. Mean heart rates also increased when compared to the pre-dose heart rate at the 10-minute time point for all groups. Heart rates returned to pre-dose rates or below at the 20 minute time points for the 2mg/kg and 6mg/kg groups, and at the 30 minute time point for the 10mg/kg group. There was a decrease in the mean respiratory rates for all treatment groups when compared to the pre-dose rate, lowest at the 10 minutes time point. Mean systolic BP decreased and was lowest in all groups at the 10 minute time point for the 2mg/kg dogs, and at the 20 minute time point for 6mg/kg and 10mg/kg groups. Similar trends were recorded for diastolic BP and MAP. Clinical pathology abnormalities were not clinically significant in all groups; abnormal necropsy and histopathology findings were associated with injection site trauma consistent with intravenous injection and repeat catheterization. No pain on injection was reported. No abnormal cardiac arrhythmias were noted during the study (ECG observed but not recorded).
Dog preanesthetic compatibility study: Forty eight healthy Beagle dogs (24 males, 24 females) were enrolled with 3 females and 3 males allocated to each of 8 preanesthetic groups (0.9% saline, medetomidine 40µg/kg, medetomidine 4µg/kg, acepromazine 1.1 mg/kg, acepromazine 0.2 mg/kg, acepromazine 0.05 mg/kg, butorphanol 0.2 mg/kg, and midazolam 0.2 mg/kg). All treatment groups received a maximum induction dose of 2 mg/kg of alfaxalone (to achieve endotracheal intubation) in conjunction with an intravenous dose of differing preanesthetic according to treatment group. No procedure was performed. Data were collected on each dog for the quality of anesthesia, as well as cardiovascular and respiratory parameters. Data for the cardiovascular and respiratory variables were collected between preanesthetic administration until recovery at intervals of -60, -5, 5, 10, 15, and 20 minutes and every 10 minutes thereafter.
Dose sparing occurred with acepromazine, medetomidine, and butorphanol. Dogs administered midazolam required an increase in dose compared to the saline group.The high medetomidine and 1.1 mg/kg acepromazine groups had the largest dose sparing effect on alfaxalone. The 0.2 mg/kg and 1.1 mg/kg acepromazine, low dose medetomidine, midazolam, and butorphanol groups had mean durations of anesthesia between 7:58 and 10:17 min/sec. The high medetomidine group had a prolonged mean duration of anesthesia at 1:10:08 (hr/min/sec). Duration of recovery increased with the duration of anesthesia. Midazolam treated dogs had the least satisfactory recovery scores.
No dog experienced hypotension. Mean heart rates decreased compared to baseline values. Dogs in the high medetomidine group experienced bradycardia through the end of anesthesia. Heart rates for the saline, 0.05 mg/kg acepromazine, and midazolam groups increased between the -5 minutes and 5-minute time points. The midazolam group experienced mean heart rates of 170-175 at the 5-10 minute time points. Dogs in the 0.2 mg/kg and 1.1 mg/kg acepromazine group, and butorphanol group had stable heart rates from baseline, premedication, and through anesthesia. Electrocardiogram recordings were evaluated by the study investigator, and no abnormal findings were noted. Blood pressures were obtained by an indirect method and remained normal in all groups throughout anesthesia. Respiratory rates decreased in the high and low medetomidine groups after premedication (-5 minutes) and again after alfaxalone administration (5 minutes). Decreases for the other groups occurred after alfaxalone administration, and had not returned to baseline values at the last recorded time point. No apnea was observed.
Dog tolerance safety study: Eight dogs (4 male, 4 female) each received 0 (0.9% saline), 2, 6, and 20 mg/kg of alfaxalone (unpreserved) in sequence, with a 3 hour washout period between doses. There were no unscheduled deaths during the study. Necropsy and histopathology were not conducted. Alfaxalone produced dose related decreases in cardiovascular, respiratory, pH, and blood gas values, and dose related increases for duration of anesthesia, time to extubation, and time to sternal recumbency. There were no ECG abnormalities reported during the study. Observations during anesthesia included forelimb rigidity and shivering/shaking during recovery, paddling, excitement during recovery, inability to intubate (1X).
Apnea occurred in a dose dependent manner, and all dogs required oxygen supplementation and positive pressure ventilation after administration of the 20mg/kg dose. One dog experienced apnea after administration of the 2mg/kg dose, and 6 dogs experienced apnea after the 6mg/kg dose. These dogs did not require oxygen supplementation. The mean duration of apnea also increased in a dose related manner. Decreases in respiratory rate were most profound at 1 through 10 minutes in the 6mg/kg group, and 1 through 30 minutes in the 20mg/kg group. Tidal volume and minute volume decreased in a dose dependent manner, along with the respiratory rate.
Blood pressures were obtained from an arterial catheter. At all doses, there was an increase in the mean heart rate, compared to baseline values. At the 20mg/kg dose, the heart rate returned to near baseline values between the 5 and 15 minute time points. At 20mg/kg, the heart rates were tachycardic (means 155-168 bpm); at the 2mg/kg and 6mg/kg doses the heart rates were elevated (means 143-150 bpm). At the 2mg/kg and 6mg/kg doses, the MAP and systolic BP increased compared to baseline, and at the 20mg/kg dose, the MAP and systolic BP decreased compared to baseline. These changes occurred at 1 and 5 minutes at the 2mg/kg dose, and at 1 minute for the 6mg/kg dose. The mean MAP and systolic BP returned to baseline values by the end of anesthesia. Cardiac output (CO) and central venous pressure (CVP) were lowest in the 20mg/kg group at 5 and 30 minutes.
Dog cesarean section safety study: Forty-eight female dogs received alfaxalone (unpreserved) for induction prior to cesarean section, and were maintained using isoflurane. The average induction dose of alfaxalone was 1.9 mg/kg. Immediate, transient, post-induction apnea occurred in 15% of cases. Cardiovascular and respiratory parameters were well maintained during induction, maintenance and recovery, and anesthesia quality was scored as good during all phases. Puppy vigor scores were rated as very good for withdrawal reflex, sucking reflex, anogenital reflex, and flexion reflex. Puppy survival rate was 96.2% at 24 hours after birth.
- STORAGE INFORMATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 mL Carton
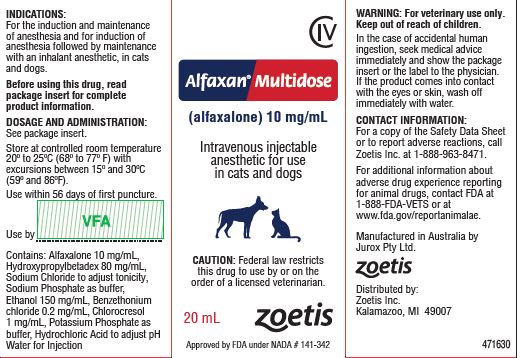
- PRINCIPAL DISPLAY PANEL - 20 mL Carton
-
INGREDIENTS AND APPEARANCE
ALFAXAN MULTIDOSE
alfaxalone injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-6696 Route of Administration INTRAVENOUS DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALFAXALONE (UNII: BD07M97B2A) (ALFAXALONE - UNII:BD07M97B2A) ALFAXALONE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYLBETADEX (0.58-0.68 MS) (UNII: 1I96OHX6EK) 80 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) ALCOHOL (UNII: 3K9958V90M) 150 mg in 1 mL BENZETHONIUM CHLORIDE (UNII: PH41D05744) 0.2 mg in 1 mL CHLOROCRESOL (UNII: 36W53O7109) 1 mg in 1 mL POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-6696-1 1 in 1 CARTON 1 10 mL in 1 VIAL, GLASS 2 NDC:54771-6696-2 1 in 1 CARTON 2 20 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141342 07/12/2018 Labeler - Zoetis Inc. (828851555)