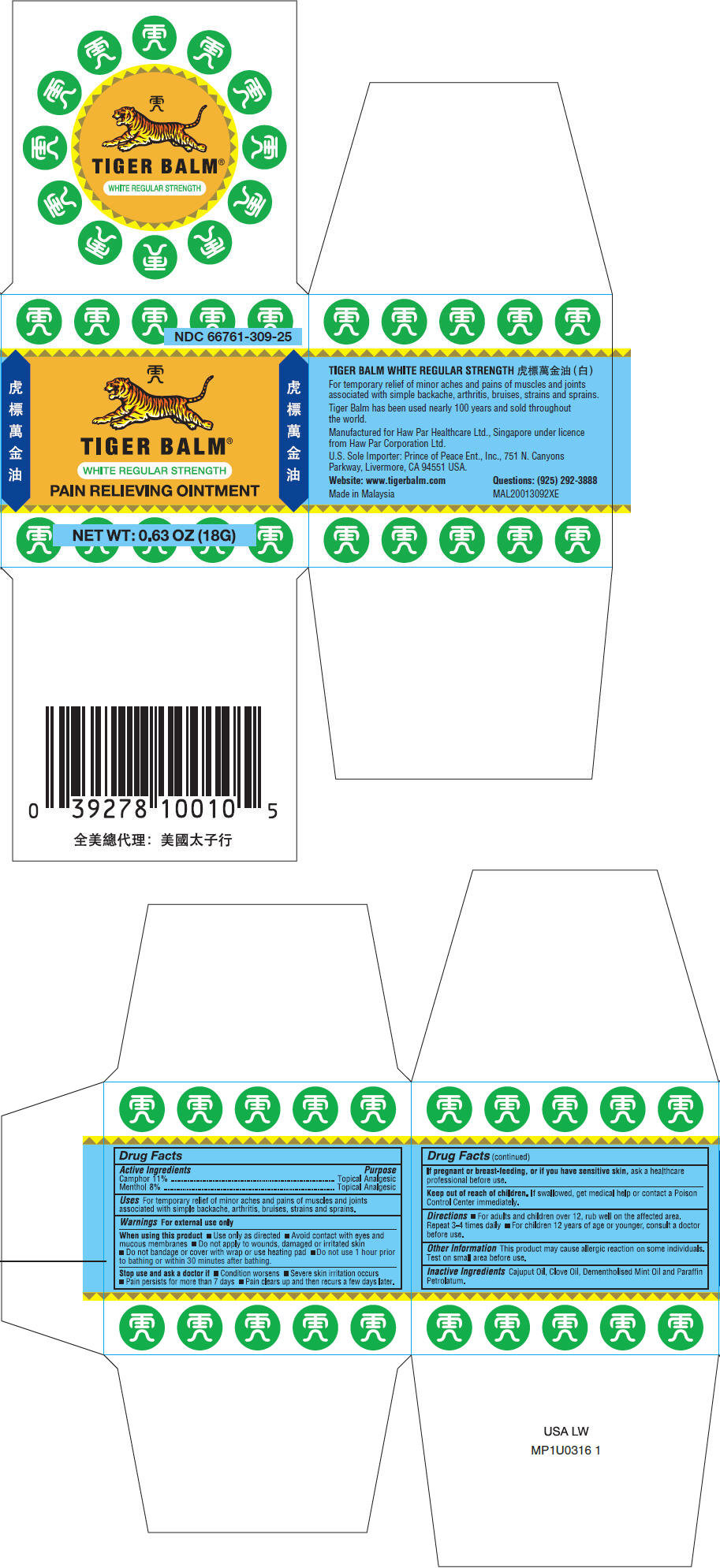
Label: TIGER BALM WHITE REGULAR STRENGTH (camphor- synthetic and menthol ointment
- NDC Code(s): 66761-309-23, 66761-309-25, 66761-309-35, 66761-309-40
- Packager: Haw Par Healthcare Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only
When using this product
- Use only as directed
- Avoid contact with eyes and mucous membranes
- Do not apply to wounds, damaged or irritated skin
- Do not bandage or cover with wrap or use heating pad
- Do not use 1 hour prior to bathing or 30 minutes after bathing.
Stop use and ask a doctor if
- Condition worsens
- Severe skin irritation occurs
- Pain persists for more that 7 days
- Pain clears up and then recurs a few days later.
- Directions
- Other Information
- Inactive Ingredients
- Questions
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 18 G Jar Box
-
INGREDIENTS AND APPEARANCE
TIGER BALM WHITE REGULAR STRENGTH
camphor (synthetic) and menthol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66761-309 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Camphor (Synthetic) (UNII: 5TJD82A1ET) (Camphor (Synthetic) - UNII:5TJD82A1ET) Camphor (Synthetic) 110 mg in 1 g Menthol, Unspecified Form (UNII: L7T10EIP3A) (Menthol, Unspecified Form - UNII:L7T10EIP3A) Menthol, Unspecified Form 80 mg in 1 g Inactive Ingredients Ingredient Name Strength Cajuput oil (UNII: J3TO6BUQ37) clove oil (UNII: 578389D6D0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66761-309-23 4 g in 1 JAR; Type 0: Not a Combination Product 02/01/2007 2 NDC:66761-309-25 1 in 1 BOX 02/01/2007 2 18 g in 1 JAR; Type 0: Not a Combination Product 3 NDC:66761-309-35 1 in 1 BOX 02/01/2007 3 18 g in 1 JAR; Type 0: Not a Combination Product 4 NDC:66761-309-40 1 in 1 BOX 03/01/2021 4 4 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/01/2007 Labeler - Haw Par Healthcare Ltd. (659207039) Establishment Name Address ID/FEI Business Operations Haw Par Healthcare Ltd. 659207039 MANUFACTURE(66761-309) Establishment Name Address ID/FEI Business Operations Tiger Balm (Malaysia) Sdn.Bhd. 652104126 MANUFACTURE(66761-309) Establishment Name Address ID/FEI Business Operations Xiamen Tiger Medical Company, Ltd. 527257083 MANUFACTURE(66761-309)