Label: WHITE FLOWER ANALGESIC BALM- camphor, menthol and methyl salicylate oil
- NDC Code(s): 48256-0002-1, 48256-0002-2, 48256-0002-3, 48256-0002-4
- Packager: HOE HIN PAK FAH YEOW MANUFACTORY LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- DO NOT USE
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive irritation of the skin develops
- nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
- pain persists for more than 10 days
- redness is present
- in conditions affecting children under 12 years of age
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- INDICATIONS & USAGE
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WHITE FLOWER ANALGESIC BALM
camphor, menthol and methyl salicylate oilProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:48256-0002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 6 g in 100 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 14.5 g in 100 mL METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 40.02 g in 100 mL Inactive Ingredients Ingredient Name Strength EUCALYPTUS OIL (UNII: 2R04ONI662) LAVENDER OIL (UNII: ZBP1YXW0H8) PEPPERMINT OIL (UNII: AV092KU4JH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48256-0002-1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 10/01/2018 2 NDC:48256-0002-2 10 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 10/01/2018 3 NDC:48256-0002-4 2.5 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 10/01/2018 4 NDC:48256-0002-3 5 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 10/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/11/2010 Labeler - HOE HIN PAK FAH YEOW MANUFACTORY LTD (686359001) Establishment Name Address ID/FEI Business Operations HOE HIN PAK FAH YEOW MFY LTD 686212961 manufacture(48256-0002)

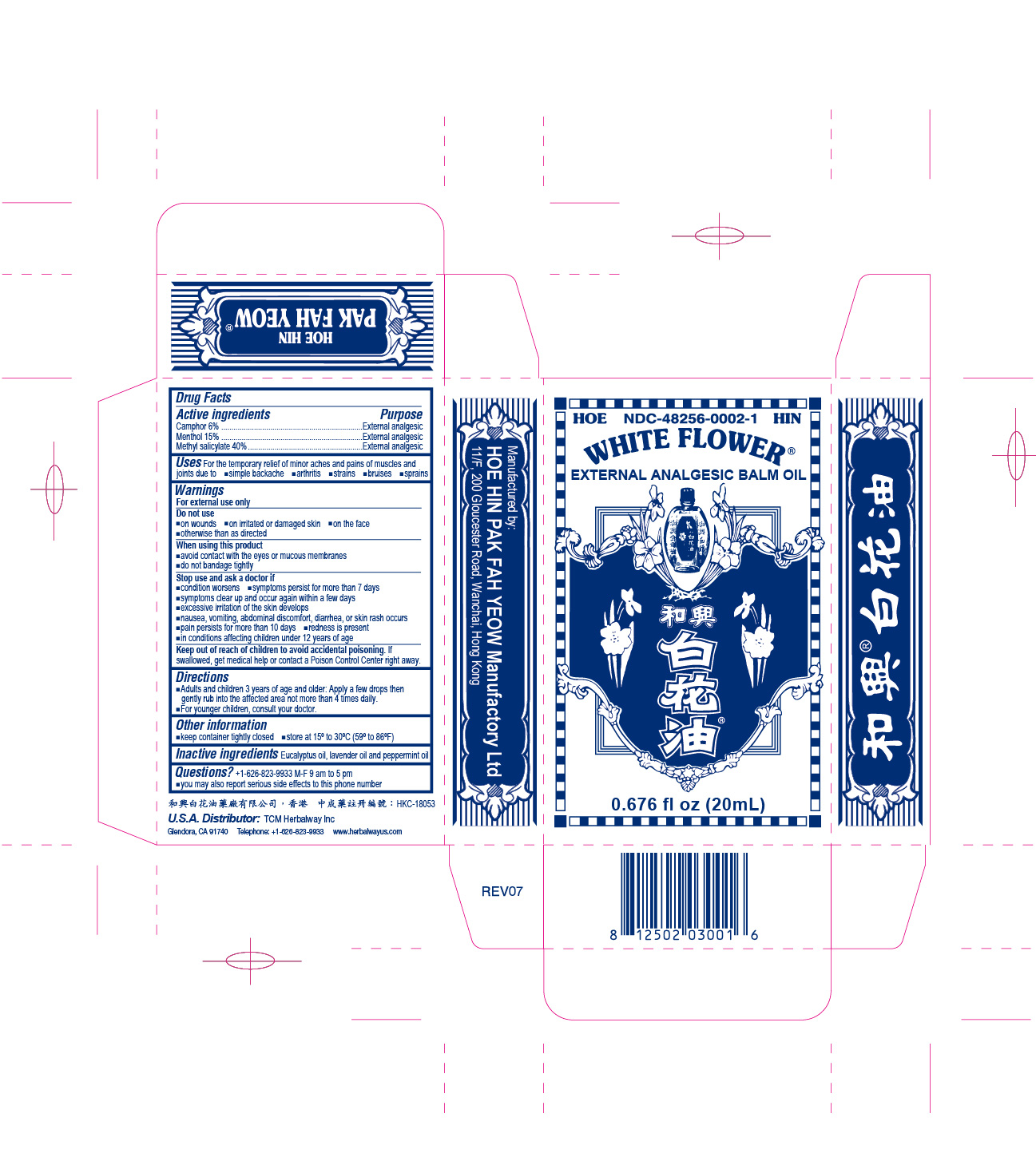
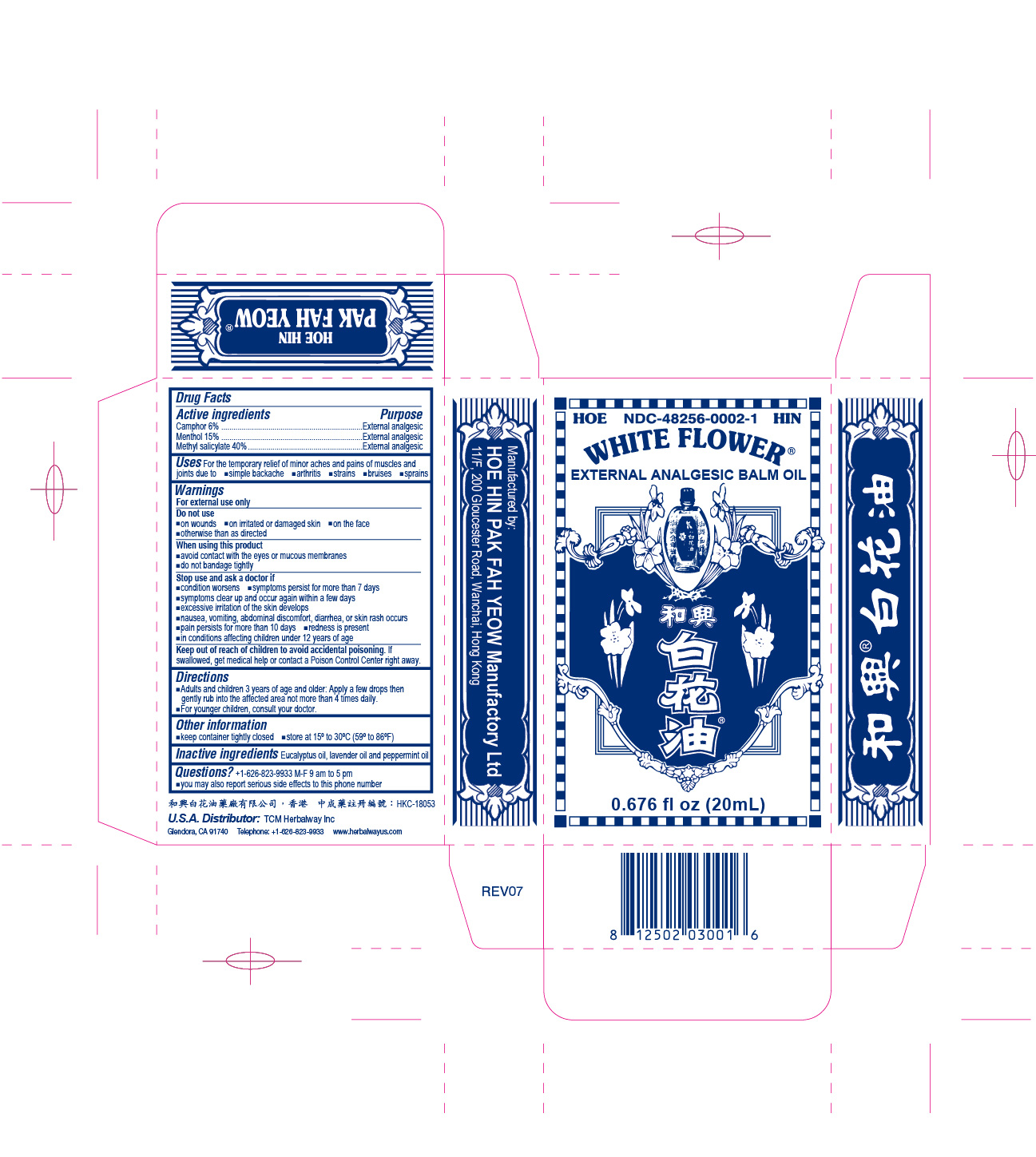 White Flower Analgesic Balm NDC-48256-0002 0.676 fl oz (20mL)
White Flower Analgesic Balm NDC-48256-0002 0.676 fl oz (20mL)