Label: GLATIRAMER ACETATE injection, solution
- NDC Code(s): 0378-6960-32, 0378-6960-93
- Packager: Mylan Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GLATIRAMER ACETATE INJECTION safely and effectively. See full prescribing information for GLATIRAMER ACETATE INJECTION.
GLATIRAMER ACETATE injection, for subcutaneous use
Initial U.S. Approval: 1996RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Glatiramer acetate injection is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults (1).
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- •
- Injection: 20 mg/mL in a single-dose, prefilled syringe with a light blue plunger (3)
CONTRAINDICATIONS
Known hypersensitivity to glatiramer acetate or mannitol (4)
WARNINGS AND PRECAUTIONS
- •
- Immediate Post-Injection Reaction (flushing, chest pain, palpitations, tachycardia, anxiety, dyspnea, throat constriction, and/or urticaria), may occur within seconds to minutes after injection and are generally transient and self-limiting (5.1)
- •
- Chest pain, usually transient (5.2)
- •
- Lipoatrophy and skin necrosis may occur. Instruct patients in proper injection technique and to rotate injection sites (5.3)
- •
- Glatiramer acetate can modify immune response (5.4)
- •
- Hepatic Injury: if signs or symptoms of hepatic dysfunction occur, consider discontinuing glatiramer acetate (5.5)
- •
- Glatiramer Acetate Products and Administration Errors: Using an optional autoinjector that is not compatible for use with Mylan’s glatiramer acetate injection may increase the risk for medication errors, such as dose omission or administration of a partial dose. (5.6)
ADVERSE REACTIONS
- •
- In controlled studies of glatiramer acetate injection 20 mg/mL, most common adverse reactions (≥ 10% and ≥ 1.5 times higher than placebo) were: injection site reactions, vasodilatation, rash, dyspnea, and chest pain (6.1)
- •
- In a controlled study of glatiramer acetate injection 40 mg/mL, most common adverse reactions (≥ 10% and ≥ 1.5 times higher than placebo) were: injection site reactions (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Instructions for Use
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Immediate Post-Injection Reaction
5.2 Chest Pain
5.3 Lipoatrophy and Skin Necrosis
5.4 Potential Effects on Immune Response
5.5 Hepatic Injury
5.6 Glatiramer Acetate Products and Administration Errors
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Patients with Impaired Renal Function
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
Glatiramer acetate injection is for subcutaneous use only [see Dosage and Administration (2.2)]. Do not administer intravenously. The dosing schedule depends on the product strength that is selected. The recommended dose is:
- •
- Glatiramer acetate injection 20 mg per mL: administer once per day
Glatiramer acetate injection 20 mg per mL and glatiramer acetate injection 40 mg per mL are not interchangeable.
2.2 Instructions for Use
Remove one blister-packaged prefilled syringe from the refrigerated carton. Let the prefilled syringe stand at room temperature for 20 minutes to allow the solution to warm to room temperature. Visually inspect the syringe for particulate matter and discoloration prior to administration. The solution in the syringe should appear clear, colorless to slightly yellow. If particulate matter or discoloration is observed, discard the syringe.
Areas for subcutaneous self-injection include arms, abdomen, hips, and thighs. The prefilled syringe is for single use only. Discard unused portions.
Using an autoinjector that is not compatible for use with Mylan’s glatiramer acetate injection may increase the risk for medication errors, such as dose omission or administration of a partial dose [see Warnings and Precautions (5.6)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Immediate Post-Injection Reaction
Approximately 16% of patients exposed to glatiramer acetate injection 20 mg per mL in the 5 placebo-controlled trials compared to 4% of those on placebo, and approximately 2% of patients exposed to glatiramer acetate injection 40 mg per mL in a placebo-controlled trial compared to none on placebo, experienced a constellation of symptoms that may occur immediately (within seconds to minutes, with the majority of symptoms observed within 1 hour) after injection and included at least two of the following: flushing, chest pain, palpitations, tachycardia, anxiety, dyspnea, constriction of the throat, and urticaria. In general, these symptoms have their onset several months after the initiation of treatment, although they may occur earlier, and a given patient may experience one or several episodes of these symptoms. Whether or not any of these symptoms actually represent a specific syndrome is uncertain. Typically, the symptoms were transient and self-limited and did not require treatment; however, there have been reports of patients with similar symptoms who received emergency medical care. Whether an immunologic or nonimmunologic mechanism mediates these episodes, or whether several similar episodes seen in a given patient have identical mechanisms, is unknown.
5.2 Chest Pain
Approximately 13% of glatiramer acetate injection 20 mg per mL patients in the 5 placebo-controlled studies compared to 6% of placebo patients, and approximately 2% of patients exposed to glatiramer acetate injection 40 mg per mL in a placebo-controlled trial compared to 1% of placebo patients, experienced at least one episode of transient chest pain. While some of these episodes occurred in the context of the Immediate Post-Injection Reaction described above, many did not. The temporal relationship of this chest pain to an injection was not always known. The pain was usually transient, often unassociated with other symptoms, and appeared to have no clinical sequelae. Some patients experienced more than one such episode, and episodes usually began at least 1 month after the initiation of treatment. The pathogenesis of this symptom is unknown.
5.3 Lipoatrophy and Skin Necrosis
At injection sites, localized lipoatrophy and, rarely, injection site skin necrosis may occur. Lipoatrophy occurred in approximately 2% of patients exposed to glatiramer acetate injection 20 mg per mL in the 5 placebo-controlled trials compared to none on placebo, and 0.5% of patients exposed to glatiramer acetate injection 40 mg per mL in a single placebo-controlled trial and none on placebo. Skin necrosis has only been observed in the postmarketing setting. Lipoatrophy may occur at various times after treatment onset (sometimes after several months) and is thought to be permanent. There is no known therapy for lipoatrophy. To assist in possibly minimizing these events, the patient should be advised to follow proper injection technique and to rotate injection sites with each injection.
5.4 Potential Effects on Immune Response
Because glatiramer acetate can modify immune response, it may interfere with immune functions. For example, treatment with glatiramer acetate may interfere with the recognition of foreign antigens in a way that would undermine the body's tumor surveillance and its defenses against infection. There is no evidence that glatiramer acetate does this, but there has not been a systematic evaluation of this risk. Because glatiramer acetate is an antigenic material, it is possible that its use may lead to the induction of host responses that are untoward, but systematic surveillance for these effects has not been undertaken.
Although glatiramer acetate is intended to minimize the autoimmune response to myelin, there is the possibility that continued alteration of cellular immunity due to chronic treatment with glatiramer acetate may result in untoward effects.
Glatiramer acetate-reactive antibodies are formed in most patients receiving glatiramer acetate. Studies in both the rat and monkey have suggested that immune complexes are deposited in the renal glomeruli. Furthermore, in a controlled trial of 125 RRMS patients given glatiramer acetate injection 20 mg per mL, subcutaneously every day for 2 years, serum IgG levels reached at least 3 times baseline values in 80% of patients by 3 months of initiation of treatment. By 12 months of treatment, however, 30% of patients still had IgG levels at least 3 times baseline values, and 90% had levels above baseline by 12 months. The antibodies are exclusively of the IgG subtype and predominantly of the IgG-1 subtype. No IgE type antibodies could be detected in any of the 94 sera tested; nevertheless, anaphylaxis can be associated with the administration of most any foreign substance, and therefore, this risk cannot be excluded.
5.5 Hepatic Injury
Cases of hepatic injury, some severe, including liver failure and hepatitis with jaundice, have been reported with glatiramer acetate. Hepatic injury has occurred from days to years after initiating treatment with glatiramer acetate. If signs or symptoms of liver dysfunction occur, consider discontinuation of glatiramer acetate.
5.6 Glatiramer Acetate Products and Administration Errors
Medication errors have occurred when glatiramer acetate products are administered with incompatible autoinjectors. Some glatiramer acetate products can be administered by an optional compatible autoinjector, should one be available; however, not all glatiramer acetate products have a marketed optional compatible autoinjector for administration [see Dosage and Administration (2.2) and How Supplied/Storage and Handling (16)].
Using an optional autoinjector that is not compatible for use with Mylan’s glatiramer acetate injection may increase the risk for medication errors, such as dose omission or administration of a partial dose.
If using an optional autoinjector for administration, ensure the device is compatible for use with the specific glatiramer acetate product by referring to the autoinjector labeling. The availability of compatible autoinjectors for each glatiramer acetate product may change with time.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- •
- Immediate Post-Injection Reaction [see Warnings and Precautions (5.1)]
- •
- Chest Pain [see Warnings and Precautions (5.2)]
- •
- Lipoatrophy and Skin Necrosis [see Warnings and Precautions (5.3)]
- •
- Potential Effects on Immune Response [see Warnings and Precautions (5.4)]
- •
- Hepatic Injury [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Incidence in Controlled Clinical Trials
Glatiramer Acetate Injection 20 mg per mL per day
Among 563 patients treated with glatiramer acetate injection in blinded placebo-controlled trials, approximately 5% of the subjects discontinued treatment because of an adverse reaction. The adverse reactions most commonly associated with discontinuation were: injection site reactions, dyspnea, urticaria, vasodilatation, and hypersensitivity. The most common adverse reactions were: injection site reactions, vasodilatation, rash, dyspnea, and chest pain.
Table 1 lists signs and symptoms that occurred in at least 2% of patients treated with glatiramer acetate injection 20 mg per mL in the placebo-controlled trials. These signs and symptoms were numerically more common in patients treated with glatiramer acetate injection than in patients treated with placebo. Adverse reactions were usually mild in intensity.
Table 1: Adverse Reactions in Controlled Clinical Trials with an Incidence ≥ 2% of Patients and More Frequent with Glatiramer Acetate Injection (20 mg per mL Daily) than with Placebo - *
- Injection site atrophy comprises terms relating to localized lipoatrophy at injection site
Glatiramer Acetate Injection
20 mg/mL
(n = 563)
%Placebo
(n = 564)
%Blood and Lymphatic System Disorders
Lymphadenopathy
7
3
Cardiac Disorders
Palpitations
9
4
Tachycardia
5
2
Eye Disorders
Eye Disorder
3
1
Diplopia
3
2
Gastrointestinal Disorders
Nausea
15
11
Vomiting
7
4
Dysphagia
2
1
General Disorders and Administration Site Conditions
Injection Site Erythema
43
10
Injection Site Pain
40
20
Injection Site Pruritus
27
4
Injection Site Mass
26
6
Asthenia
22
21
Pain
20
17
Injection Site Edema
19
4
Chest Pain
13
6
Injection Site Inflammation
9
1
Edema
8
2
Injection Site Reaction
8
1
Pyrexia
6
5
Injection Site Hypersensitivity
4
0
Local Reaction
3
1
Chills
3
1
Face Edema
3
1
Edema Peripheral
3
2
Injection Site Fibrosis
2
1
Injection Site Atrophy*
2
0
Immune System Disorders
Hypersensitivity
3
2
Infections and Infestations
Infection
30
28
Influenza
14
13
Rhinitis
7
5
Bronchitis
6
5
Gastroenteritis
6
4
Vaginal Candidiasis
4
2
Metabolism and Nutrition Disorders
Weight Increased
3
1
Musculoskeletal and Connective Tissue Disorders
Back Pain
12
10
Neoplasms Benign, Malignant and Unspecified (Incl Cysts and Polyps)
Benign Neoplasm of Skin
2
1
Nervous System Disorders
Tremor
4
2
Migraine
4
2
Syncope
3
2
Speech Disorder
2
1
Psychiatric Disorders
Anxiety
13
10
Nervousness
2
1
Renal and Urinary Disorders
Micturition Urgency
5
4
Respiratory, Thoracic and Mediastinal Disorders
Dyspnea
14
4
Cough
6
5
Laryngospasm
2
1
Skin and Subcutaneous Tissue Disorders
Rash
19
11
Hyperhidrosis
7
5
Pruritus
5
4
Urticaria
3
1
Skin Disorder
3
1
Vascular Disorders
Vasodilatation
20
5
Adverse reactions which occurred only in 4 to 5 more subjects in the glatiramer acetate group than in the placebo group (less than 1% difference), but for which a relationship to glatiramer acetate could not be excluded, were arthralgia and herpes simplex.
Laboratory analyses were performed on all patients participating in the clinical program for glatiramer acetate. Clinically-significant laboratory values for hematology, chemistry, and urinalysis were similar for both glatiramer acetate and placebo groups in blinded clinical trials. In controlled trials one patient discontinued treatment due to thrombocytopenia (16 x109/L), which resolved after discontinuation of treatment.
Data on adverse reactions occurring in the controlled clinical trials of glatiramer acetate injection 20 mg per mL were analyzed to evaluate differences based on sex. No clinically-significant differences were identified. Ninety-six percent of patients in these clinical trials were Caucasian. The majority of patients treated with glatiramer acetate injection were between the ages of 18 and 45. Consequently, data are inadequate to perform an analysis of the adverse reaction incidence related to clinically-relevant age subgroups.
Other Adverse Reactions
In the paragraphs that follow, the frequencies of less commonly reported adverse clinical reactions are presented. Because the reports include reactions observed in open and uncontrolled premarketing studies (n = 979), the role of glatiramer acetate in their causation cannot be reliably determined. Furthermore, variability associated with adverse reaction reporting, the terminology used to describe adverse reactions, etc., limit the value of the quantitative frequency estimates provided. Reaction frequencies are calculated as the number of patients who used glatiramer acetate and reported a reaction divided by the total number of patients exposed to glatiramer acetate. All reported reactions are included except those already listed in the previous table, those too general to be informative, and those not reasonably associated with the use of the drug. Reactions are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: Frequent adverse reactions are defined as those occurring in at least 1/100 patients and infrequent adverse reactions are those occurring in 1/100 to 1/1,000 patients.
Body as a Whole:
Frequent: Abscess.
Infrequent: Injection site hematoma, moon face, cellulitis, hernia, injection site abscess, serum sickness, suicide attempt, injection site hypertrophy, injection site melanosis, lipoma, and photosensitivity reaction.
Cardiovascular:
Frequent: Hypertension.
Infrequent: Hypotension, midsystolic click, systolic murmur, atrial fibrillation, bradycardia, fourth heart sound, postural hypotension, and varicose veins.
Digestive:
Infrequent: Dry mouth, stomatitis, burning sensation on tongue, cholecystitis, colitis, esophageal ulcer, esophagitis, gastrointestinal carcinoma, gum hemorrhage, hepatomegaly, increased appetite, melena, mouth ulceration, pancreas disorder, pancreatitis, rectal hemorrhage, tenesmus, tongue discoloration, and duodenal ulcer.
Endocrine:
Infrequent: Goiter, hyperthyroidism, and hypothyroidism.
Gastrointestinal:
Frequent: Bowel urgency, oral moniliasis, salivary gland enlargement, tooth caries, and ulcerative stomatitis.
Hemic and Lymphatic:
Infrequent: Leukopenia, anemia, cyanosis, eosinophilia, hematemesis, lymphedema, pancytopenia, and splenomegaly.
Metabolic and Nutritional:
Infrequent: Weight loss, alcohol intolerance, Cushing’s syndrome, gout, abnormal healing, and xanthoma.
Musculoskeletal:
Infrequent: Arthritis, muscle atrophy, bone pain, bursitis, kidney pain, muscle disorder, myopathy, osteomyelitis, tendon pain, and tenosynovitis.
Nervous:
Frequent: Abnormal dreams, emotional lability, and stupor.
Infrequent: Aphasia, ataxia, convulsion, circumoral paresthesia, depersonalization, hallucinations, hostility, hypokinesia, coma, concentration disorder, facial paralysis, decreased libido, manic reaction, memory impairment, myoclonus, neuralgia, paranoid reaction, paraplegia, psychotic depression, and transient stupor.
Respiratory:
Frequent: Hyperventilation and hay fever.
Infrequent: Asthma, pneumonia, epistaxis, hypoventilation, and voice alteration.
Skin and Appendages:
Frequent: Eczema, herpes zoster, pustular rash, skin atrophy, and warts.
Infrequent: Dry skin, skin hypertrophy, dermatitis, furunculosis, psoriasis, angioedema, contact dermatitis, erythema nodosum, fungal dermatitis, maculopapular rash, pigmentation, benign skin neoplasm, skin carcinoma, skin striae, and vesiculobullous rash.
Special Senses:
Frequent: Visual field defect.
Infrequent: Dry eyes, otitis externa, ptosis, cataract, corneal ulcer, mydriasis, optic neuritis, photophobia, and taste loss.
Urogenital:
Frequent: Amenorrhea, hematuria, impotence, menorrhagia, suspicious papanicolaou smear, urinary frequency, and vaginal hemorrhage.
Infrequent: Vaginitis, flank pain (kidney), breast engorgement, breast enlargement, carcinoma in situ cervix, fibrocystic breast, kidney calculus, nocturia, ovarian cyst, priapism, pyelonephritis, abnormal sexual function, and urethritis.
Glatiramer Acetate Injection 40 mg per mL three times per week
Among 943 patients treated with glatiramer acetate injection 40 mg per mL three times per week in a blinded, placebo-controlled trial, approximately 3% of the subjects discontinued treatment because of an adverse reaction. The most common adverse reactions were injection site reactions, which were also the most common cause of discontinuation.
Table 2 lists signs and symptoms that occurred in at least 2% of patients treated with glatiramer acetate injection 40 mg per mL in the blinded, placebo-controlled trial. These signs and symptoms were numerically more common in patients treated with glatiramer acetate injection 40 mg per mL than in patients treated with placebo. Adverse reactions were usually mild in intensity.
Table 2: Adverse Reactions in a Controlled Clinical Trial with an Incidence ≥ 2% of Patients and More Frequent with Glatiramer Acetate Injection (40 mg per mL Three Times per Week) than with Placebo Glatiramer Acetate
Injection
40 mg/mL
(n = 943)
%Placebo
(n = 461)
%General Disorders and Administration Site Conditions
Injection Site Erythema
22
2
Injection Site Pain
10
2
Injection Site Mass
6
0
Injection Site Pruritus
6
0
Injection Site Edema
6
0
Pyrexia
3
2
Influenza-like Illness
3
2
Injection Site Inflammation
2
0
Chills
2
0
Chest Pain
2
1
Infections and Infestations
Nasopharyngitis
11
9
Respiratory Tract Infection Viral
3
2
Respiratory, Thoracic and Mediastinal Disorders
Dyspnea
3
0
Vascular Disorders
Vasodilatation
3
0
Gastrointestinal Disorders
Nausea
2
1
Skin and Subcutaneous Tissue Disorders
Erythema
2
0
Rash
2
1
No new adverse reactions appeared in subjects treated with glatiramer acetate injection 40 mg per mL three times per week as compared to subjects treated with glatiramer acetate injection 20 mg per mL per day in clinical trials and during postmarketing experience. Data on adverse reactions occurring in the controlled clinical trial of glatiramer acetate injection 40 mg per mL were analyzed to evaluate differences based on sex. No clinically significant differences were identified. Ninety-eight percent of patients in this clinical trial were Caucasian and the majority were between the ages of 18 and 50. Consequently, data are inadequate to perform an analysis of the adverse reaction incidence related to clinically-relevant age groups.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of glatiramer acetate injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: sepsis; SLE syndrome; hydrocephalus; enlarged abdomen; allergic reaction; anaphylactoid reaction
Cardiovascular System: thrombosis; peripheral vascular disease; pericardial effusion; myocardial infarct; deep thrombophlebitis; coronary occlusion; congestive heart failure; cardiomyopathy; cardiomegaly; arrhythmia; angina pectoris
Digestive System: tongue edema; stomach ulcer; hemorrhage; eructation
Hemic and Lymphatic System: thrombocytopenia; lymphoma-like reaction; acute leukemia
Hepatobiliary Disorders: cholelithiasis; liver function abnormality; cirrhosis of the liver; hepatitis; hepatic injury [see Warnings and Precautions (5.5)]
Metabolic and Nutritional Disorders: hypercholesterolemia
Musculoskeletal System: rheumatoid arthritis; generalized spasm
Nervous System: myelitis; meningitis; CNS neoplasm; cerebrovascular accident; brain edema; abnormal dreams; aphasia; convulsion; neuralgia
Respiratory System: pulmonary embolus; pleural effusion; carcinoma of lung
Special Senses: glaucoma; blindness
Urogenital System: urogenital neoplasm; urine abnormality; ovarian carcinoma; nephrosis; kidney failure; breast carcinoma; bladder carcinoma; urinary frequency
-
7 DRUG INTERACTIONS
Interactions between glatiramer acetate and other drugs have not been fully evaluated. Results from existing clinical trials do not suggest any significant interactions of glatiramer acetate with therapies commonly used in MS patients, including the concurrent use of corticosteroids for up to 28 days. Glatiramer acetate has not been formally evaluated in combination with interferon beta.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available human data on the use of glatiramer acetate injection in pregnant women are not sufficient to support conclusions about drug-associated risk for major birth defects and miscarriage. Administration of glatiramer acetate by subcutaneous injection to pregnant rats and rabbits resulted in no adverse effects on embryofetal or offspring development (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
There are no adequate and well-controlled studies of glatiramer acetate injection in pregnant women. The available postmarketing reports, case series, and small cohort studies do not provide sufficient information to support conclusions about drug-associated risk for major birth defects and miscarriage.
Animal Data
In rats or rabbits receiving glatiramer acetate by subcutaneous injection during the period of organogenesis, no adverse effects on embryofetal development were observed at doses up to 37.5 mg/kg/day (18 and 36 times, respectively, the therapeutic human dose of 20 mg/day on a mg/m2 basis). In rats receiving subcutaneous glatiramer acetate at doses of up to 36 mg/kg from day 15 of pregnancy throughout lactation, no significant effects on delivery or on offspring growth and development were observed.
8.2 Lactation
Risk Summary
There are no data on the presence of glatiramer acetate in human milk. Based on the low systemic exposure because of substantial local hydrolysis of glatiramer acetate following subcutaneous administration, breastfeeding is not expected to result in clinically relevant exposure of the infant to the drug [see Clinical Pharmacology (12.3)]. There are no data on the effects of glatiramer acetate on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for glatiramer acetate injection and any potential adverse effects on the breastfed infant from glatiramer acetate injection or from the underlying maternal condition.
-
11 DESCRIPTION
Glatiramer acetate, the active ingredient of glatiramer acetate injection, consists of the acetate salts of synthetic polypeptides, containing four naturally occurring amino acids: L-glutamic acid, L-alanine, L-tyrosine, and L-lysine with an average molar fraction of 0.141, 0.427, 0.095, and 0.338, respectively. The average molecular weight of glatiramer acetate is 5,000 – 9,000 daltons. Glatiramer acetate is identified by specific antibodies.
Chemically, glatiramer acetate is designated L-glutamic acid polymer with L-alanine, L-lysine and L-tyrosine, acetate (salt). Its structural formula is:
(Glu, Ala, Lys, Tyr)x ●xCH3COOH
(C5H9NO4●C3H7NO2●C6H14N2O2●C9H11NO3)x ●xC2H4O2
CAS - 147245-92-9Glatiramer acetate is a clear, colorless to slightly yellow, sterile, nonpyrogenic solution for subcutaneous injection. Each 1 mL of glatiramer acetate solution contains 20 mg of glatiramer acetate and the following inactive ingredient: 40 mg of mannitol. The pH of the solutions is approximately 5.5 to 7.0. The biological activity of glatiramer acetate is determined by its ability to block the induction of experimental autoimmune encephalomyelitis (EAE) in mice.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism(s) by which glatiramer acetate exerts its effects in patients with MS are not fully understood. However, glatiramer acetate is thought to act by modifying immune processes that are believed to be responsible for the pathogenesis of MS. This hypothesis is supported by findings of studies that have been carried out to explore the pathogenesis of experimental autoimmune encephalomyelitis, a condition induced in animals through immunization against central nervous system derived material containing myelin and often used as an experimental animal model of MS. Studies in animals and in vitro systems suggest that upon its administration, glatiramer acetate-specific suppressor T-cells are induced and activated in the periphery.
Because glatiramer acetate can modify immune functions, concerns exist about its potential to alter naturally-occurring immune responses. There is no evidence that glatiramer acetate does this, but this has not been systematically evaluated [see Warnings and Precautions (5.4)].
12.3 Pharmacokinetics
Results obtained in pharmacokinetic studies performed in humans (healthy volunteers) and animals support that a substantial fraction of the therapeutic dose delivered to patients subcutaneously is hydrolyzed locally. Larger fragments of glatiramer acetate can be recognized by glatiramer acetate-reactive antibodies. Some fraction of the injected material, either intact or partially hydrolyzed, is presumed to enter the lymphatic circulation, enabling it to reach regional lymph nodes, and some may enter the systemic circulation intact.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 2-year carcinogenicity study, mice were administered up to 60 mg/kg/day glatiramer acetate by subcutaneous injection (up to 15 times the human therapeutic dose of 20 mg/day on a mg/m2 basis). No increase in systemic neoplasms was observed. In males receiving the 60-mg/kg/day dose, there was an increased incidence of fibrosarcomas at the injection sites. These sarcomas were associated with skin damage precipitated by repetitive injections of an irritant over a limited skin area.
In a 2-year carcinogenicity study, rats were administered up to 30 mg/kg/day glatiramer acetate by subcutaneous injection (up to 15 times the human therapeutic dose on a mg/m2 basis). No increase in neoplasms was observed.
Mutagenesis
Glatiramer acetate was not mutagenic in in vitro (Ames test, mouse lymphoma tk) assays. Glatiramer acetate was clastogenic in two separate in vitro chromosomal aberration assays in cultured human lymphocytes but not clastogenic in an in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
When glatiramer acetate was administered by subcutaneous injection prior to and during mating (males and females) and throughout gestation and lactation (females) at doses up to 36 mg/kg/day (18 times the human therapeutic dose on a mg/m2 basis) no adverse effects were observed on reproductive or developmental parameters.
-
14 CLINICAL STUDIES
Evidence supporting the effectiveness of glatiramer acetate derives from five placebo-controlled trials, four of which used a glatiramer acetate injection dose of 20 mg per mL per day and one of which used a glatiramer acetate injection dose of 40 mg per mL three times per week.
Glatiramer Acetate Injection 20 mg per mL per day: Study 1 was performed at a single center. Fifty patients were enrolled and randomized to receive daily doses of either glatiramer acetate injection, 20 mg per mL subcutaneously, or placebo (glatiramer acetate injection: n = 25; placebo: n = 25). Patients were diagnosed with RRMS by standard criteria, and had at least 2 exacerbations during the 2 years immediately preceding enrollment. Patients were ambulatory, as evidenced by a score of no more than 6 on the Kurtzke Disability Scale Score (DSS), a standard scale ranging from 0–Normal to 10–Death due to MS. A score of 6 is defined as one at which a patient is still ambulatory with assistance; a score of 7 means the patient must use a wheelchair.
Patients were examined every 3 months for 2 years, as well as within several days of a presumed exacerbation. To confirm an exacerbation, a blinded neurologist had to document objective neurologic signs, as well as document the existence of other criteria (e.g., the persistence of the neurological signs for at least 48 hours).
The protocol-specified primary outcome measure was the proportion of patients in each treatment group who remained exacerbation free for the 2 years of the trial, but two other important outcomes were also specified as endpoints: the frequency of attacks during the trial, and the change in the number of attacks compared with the number which occurred during the previous 2 years.
Table 3 presents the values of the three outcomes described above, as well as several protocol-specified secondary measures. These values are based on the intent-to-treat population (i.e., all patients who received at least 1 dose of treatment and who had at least 1 on-treatment assessment):
Table 3: Study 1 Efficacy Results - *
- Progression was defined as an increase of at least 1 point on the DSS, persisting for at least 3 consecutive months.
Glatiramer Acetate Injection
20 mg/mL
(n = 25)
Placebo
(n = 25)
P-Value
% Relapse-Free Patients
14/25 (56%)
7/25 (28%)
0.085
Mean Relapse Frequency
0.6/2 years
2.4/2 years
0.005
Reduction in Relapse Rate Compared to Prestudy
3.2
1.6
0.025
Median Time to First Relapse (days)
> 700
150
0.03
% of Progression-Free* Patients
20/25 (80%)
13/25 (52%)
0.07
Study 2 was a multicenter trial of similar design which was performed in 11 US centers. A total of 251 patients (glatiramer acetate injection: n = 125; placebo: n = 126) were enrolled. The primary outcome measure was the Mean 2-Year Relapse Rate. Table 4 presents the values of this outcome for the intent-to-treat population, as well as several secondary measures:
Table 4: Study 2 Efficacy Results Glatiramer Acetate Injection
20 mg/mL
(n = 125)
Placebo
(n = 126)
P-Value
Mean No. of Relapses
1.19/2 years
1.68/2 years
0.055
% Relapse-Free Patients
42/125 (34%)
34/126 (27%)
0.25
Median Time to First Relapse (days)
287
198
0.23
% of Progression-Free Patients
98/125 (78%)
95/126 (75%)
0.48
Mean Change in DSS
-0.05
+0.21
0.023
In both studies, glatiramer acetate exhibited a clear beneficial effect on relapse rate, and it is based on this evidence that glatiramer acetate is considered effective.
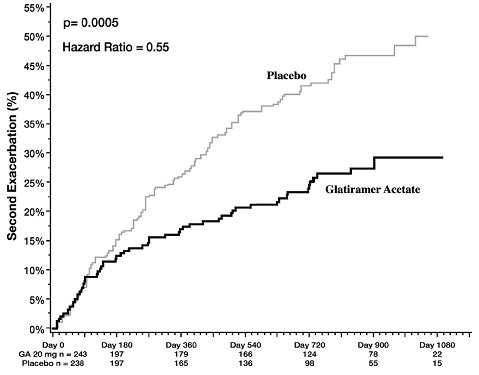
In Study 3, 481 patients who had recently (within 90 days) experienced an isolated demyelinating event and who had lesions typical of multiple sclerosis on brain MRI were randomized to receive either glatiramer acetate injection 20 mg per mL (n = 243) or placebo (n = 238). The primary outcome measure was time to development of a second exacerbation. Patients were followed for up to three years or until they reached the primary endpoint. Secondary outcomes were brain MRI measures, including number of new T2 lesions and T2 lesion volume.
Time to development of a second exacerbation was significantly delayed in patients treated with glatiramer acetate injection compared to placebo (Hazard Ratio = 0.55; 95% confidence interval 0.40 to 0.77; Figure 1). The Kaplan-Meier estimates of the percentage of patients developing a relapse within 36 months were 42.9% in the placebo group and 24.7% in the glatiramer acetate group.
Patients treated with glatiramer acetate injection demonstrated fewer new T2 lesions at the last observation (rate ratio 0.41; confidence interval 0.28 to 0.59; p < 0.0001). Additionally, baseline-adjusted T2 lesion volume at the last observation was lower for patients treated with glatiramer acetate injection (ratio of 0.89; confidence interval 0.84 to 0.94; p = 0.0001).
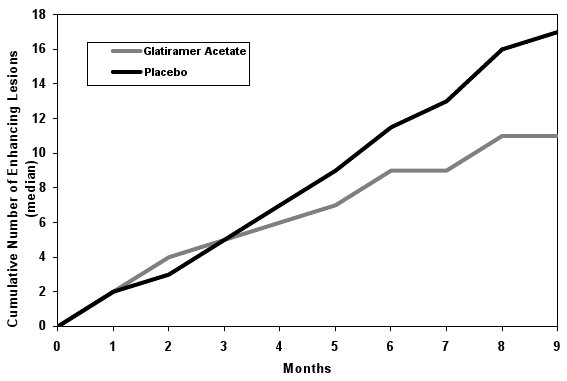
Study 4 was a multinational study in which MRI parameters were used both as primary and secondary endpoints. A total of 239 patients with RRMS (glatiramer acetate: n = 119; and placebo: n = 120) were randomized. Inclusion criteria were similar to those in the second study with the additional criterion that patients had to have at least one Gd-enhancing lesion on the screening MRI. The patients were treated in a double-blind manner for nine months, during which they underwent monthly MRI scanning. The primary endpoint for the double-blind phase was the total cumulative number of T1 Gd-enhancing lesions over the nine months. Table 5 summarizes the results for the primary outcome measure monitored during the trial for the intent-to-treat cohort.
Table 5: Study 4 MRI Results Glatiramer Acetate Injection
20 mg/mL
(n = 119)
Placebo
(n = 120)
P-Value
Medians of the Cumulative Number of T1 Gd-Enhancing Lesions
11
17
0.0030
Figure 2 displays the results of the primary outcome on a monthly basis.
Glatiramer Acetate Injection 40 mg per mL three times per week: Study 5 was a double-blind, placebo-controlled, multinational study with a total of 1404 patients with RRMS randomized in a 2:1 ratio to receive either glatiramer acetate injection 40 mg per mL (n = 943) or placebo (n = 461) three times a week for 12 months. Patients had a median of 2 relapses in the 2 years prior to screening and had not received any interferon-beta for at least 2 months prior to screening. Baseline EDSS scores ranged from 0 to 5.5 with a median of 2.5. Neurological evaluations were performed at baseline, every three months, and at unscheduled visits for suspected relapse or early termination. MRI was performed at baseline, months 6 and 12, or early termination. A total of 91% of those assigned to glatiramer acetate and 93% of those assigned to placebo completed treatment at 12 months.
The primary outcome measure was the total number of confirmed relapses (persistence of neurological symptoms for at least 48 hours confirmed on examination with objective signs). The effect of glatiramer acetate on several magnetic resonance imaging (MRI) variables, including number of new or enlarging T2 lesions and number of enhancing lesions on T1-weighted images, was also measured at months 6 and 12.
Table 6 presents the results for the intent-to-treat population.
Table 6: Study 5 Efficacy and MRI Results Glatiramer Acetate Injection
40 mg/mL
(n = 943)
Placebo
(n = 461)
P-Value
Clinical Endpoints
Number of confirmed relapses during the 12-month placebo-controlled phase
Adjusted Mean Estimates Relative risk reduction
0.331
34%
0.505
< 0.0001
MRI Endpoints
Cumulative number of new or enlarging T2 lesions at Months 6 and 12
Adjusted Mean Estimates Relative risk reduction
3.650
35%
5.592
< 0.0001
Cumulative number of enhancing lesions on T1-weighted images at Months 6 and 12
Adjusted Mean Estimates Relative risk reduction
0.905
45%
1.639
< 0.0001
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Glatiramer Acetate Injection is a clear, colorless to slightly yellow, sterile, nonpyrogenic solution in a 1 mL single-dose, prefilled syringe with fixed ½ inch 29 gauge needle supplied as:
- •
- 20 mg/mL in a single-dose, prefilled syringe with a light blue plunger, in individual blister packages supplied in 30-count cartons (NDC 0378-6960-93).
Some glatiramer acetate products can be administered by an optional compatible autoinjector. Compatible autoinjectors are supplied separately if available, but the availability of compatible autoinjectors may change with time [see Warnings and Precautions (5.6) and Patient Counseling Information (17)].
Store glatiramer acetate injection refrigerated at 2° to 8°C (36° to 46°F). If needed, the patient may store glatiramer acetate injection at room temperature, 15° to 30°C (59° to 86°F), for up to one month, but refrigeration is preferred. Avoid exposure to higher temperatures or intense light. Do not freeze glatiramer acetate injection. If a glatiramer acetate syringe freezes, it should be discarded.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Important Administration Instructions: Advise patients with new or existing glatiramer acetate prescriptions to consult their pharmacist or healthcare provider if they would like information about using an optional compatible autoinjector device, if available.
Additionally, advise patients who would like to use an autoinjector for administration, should one be available, that not all available autoinjectors are compatible with all glatiramer acetate products and the availability of compatible autoinjectors may change with time. If you have questions about the availability or compatibility of an autoinjector, contact the manufacturer of the prescribed glatiramer acetate product for more information.
Advise patients that using an optional autoinjector that is not compatible with the glatiramer acetate product may increase the risk for medication errors, such as missing a dose or administration of a partial dose [see Warnings and Precautions (5.6)].
Immediate Post-Injection Reaction: Advise patients that glatiramer acetate may cause various symptoms after injection, including flushing, chest pain, palpitations, tachycardia, anxiety, dyspnea, constriction of the throat, and urticaria. These symptoms occur within seconds to minutes after injection and are generally transient and self-limited and do not require specific treatment. Inform patients that these symptoms may occur early or may have their onset several months after the initiation of treatment. A patient may experience one or several episodes of these symptoms.
Chest Pain: Advise patients that they may experience transient chest pain either as part of the Immediate Post-Injection Reaction or in isolation. Inform patients that the pain should be transient. Some patients may experience more than one such episode, usually beginning at least one month after the initiation of treatment. Patients should be advised to seek medical attention if they experience chest pain of unusual duration or intensity.
Lipoatrophy and Skin Necrosis at Injection Site: Advise patients that localized lipoatrophy, and rarely, skin necrosis may occur at injection sites. Instruct patients to follow proper injection technique and to rotate injection areas and sites with each injection to minimize these risks.
Hepatic Injury: Advise patients that hepatic injury, including hepatic failure and hepatitis with jaundice, has been reported with the use of glatiramer acetate injection. Educate patients about the signs and symptoms of hepatic injury and instruct patients to report them immediately to their healthcare provider [see Warnings and Precautions (5.5)].
Pregnancy: Instruct patients that if they are pregnant or plan to become pregnant while taking glatiramer acetate injection they should inform their physician [see Use in Specific Populations (8.1)].
Lactation: Advise patients to notify their healthcare provider if they are breastfeeding or intend to breastfeed during glatiramer acetate injection therapy [see Use in Specific Populations (8.2)].
Instructions for Use: Instruct patients to read the glatiramer acetate injection Patient Information leaflet carefully. Glatiramer acetate injection 20 mg per mL and glatiramer acetate injection 40 mg per mL are not interchangeable. Glatiramer acetate injection 20 mg per mL is administered daily. Caution patients to use aseptic technique. The first injection should be performed under the supervision of a health care professional. Instruct patients to rotate injection areas and sites with each injection. Caution patients against the reuse of needles or syringes. Instruct patients in safe disposal procedures.
Storage Conditions: Advise patients that the recommended storage condition for glatiramer acetate injection is refrigeration at 2˚ to 8˚C (36˚ to 46˚F). If needed, the patient may store glatiramer acetate injection at room temperature, 15˚ to 30˚C (59˚ to 86˚F), for up to one month, but refrigeration is preferred. Glatiramer acetate injection should not be exposed to higher temperatures or intense light. Do not freeze glatiramer acetate injection.
-
Patient Information
Glatiramer Acetate Injection
(gla tir′ a mer as′ e tate)
for subcutaneous useRead this Patient Information before you start using glatiramer acetate injection and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is glatiramer acetate injection?
Glatiramer acetate injection is a prescription medicine that is used to treat relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
It is not known if glatiramer acetate injection is safe and effective in children under 18 years of age.
Do not take glatiramer acetate injection:
- •
- if you are allergic to glatiramer acetate or mannitol. See the end of this leaflet for a complete list of the ingredients in glatiramer acetate injection.
Before you use glatiramer acetate injection, tell your healthcare provider about all of your medical conditions, including if you:
- •
- are pregnant or plan to become pregnant. It is not known if glatiramer acetate will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if glatiramer acetate passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using glatiramer acetate injection.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Glatiramer acetate injection may affect the way other medicines work, and other medicines may affect how glatiramer acetate injection works.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine.
How should I use glatiramer acetate injection?
- •
- For detailed instructions, see the Instructions for Use at the end of this leaflet for complete information on how to use glatiramer acetate injection.
- •
- Your healthcare provider will tell you how much glatiramer acetate injection to use and when to use it.
- •
- Glatiramer acetate injection is given by injection under your skin (subcutaneously).
- •
- Use glatiramer acetate injection exactly as your healthcare provider tells you to use it.
- •
- Since every body type is different, talk with your healthcare provider about the injection areas that are best for you.
- •
- You should receive your first dose of glatiramer acetate injection with a healthcare provider or nurse present. This might be at your healthcare provider’s office or with a visiting home health nurse who will teach you how to give your glatiramer acetate injections.
- •
- Some glatiramer acetate products can be used with an optional compatible autoinjector. Compatible autoinjectors are supplied separately if available, but the availability of compatible autoinjectors may change with time.
- o
- Check with your healthcare provider when you fill or refill your medicine to make sure the autoinjector you have is meant to be used with your glatiramer acetate product. Not all optional autoinjectors are meant to be used with all glatiramer acetate products. If you use the wrong autoinjector, you might not get the correct dose of your medicine. Contact the manufacturer of your glatiramer acetate product to find out if there is an autoinjector that is meant to be used with your glatiramer acetate product.
- •
- Read your Instructions for Use and talk to your healthcare provider about the best way for you to use glatiramer acetate injection.
What are the possible side effects of glatiramer acetate injection?
Glatiramer acetate injection may cause serious side effects, including:
- •
- Immediate Post-Injection Reactions. Serious side effects may happen right after or within minutes after you inject glatiramer acetate injection at any time during your course of treatment. Call your healthcare provider right away if you have any of these immediate post-injection reaction symptoms including:
- o
- redness to your cheeks or other parts of the body (flushing)
- o
- chest pain
- o
- fast heartbeat
- o
- anxiety
- o
- breathing problems or tightness in your
throat - o
- swelling, rash, hives, or itching
If you have symptoms of an immediate post-injection reaction, do not give yourself more injections until a healthcare provider tells you to.
- •
- Chest Pain. You can have chest pain as part of an immediate post-injection reaction or by itself. This type of chest pain usually lasts a few minutes and can begin around 1 month after you start using glatiramer acetate injection. Call your healthcare provider right away if you have chest pain while using glatiramer acetate injection.
- •
-
Damage to your skin. Damage to the fatty tissue just under your skin’s surface (lipoatrophy) and, rarely, death of your skin tissue (necrosis) can happen when you use glatiramer acetate injection. Damage to the fatty tissue under your skin can cause a “dent” at the injection site that may not go away. You can reduce your chance of developing these problems by:
- o
- following your healthcare provider’s instructions for how to use glatiramer acetate injection
- o
- choosing a different injection area each time you use glatiramer acetate injection. See Step 4 in the Instructions for Use, “Choose your injection area”.
- •
- Liver problems. Liver problems, including liver failure, can occur with glatiramer acetate injection. Call your healthcare provider right away if you have symptoms, such as:
- o
- nausea
- o
- loss of appetite
- o
- tiredness
- o
- dark colored urine and pale stools
- o
- yellowing of your skin or the white part
of your eye - o
- bleeding more easily than normal
- o
- confusion
- o
- sleepiness
The most common side effects of glatiramer acetate injection are:
- •
- skin problems at your injection site, including:
- o
- redness
- o
- pain
- o
- swelling
- o
- lumps
- o
- itching
- •
- rash
- •
- shortness of breath
- •
- flushing (vasodilation)
- •
- chest pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of glatiramer acetate injection. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store glatiramer acetate injection?
- •
- Store glatiramer acetate injection in the refrigerator between 2° to 8°C (36° to 46°F).
- •
- When you are not able to refrigerate glatiramer acetate injection, you may store it for up to 1 month at room temperature between 15° to 30°C (59° to 86°F).
- •
- Protect glatiramer acetate injection from light or high temperature.
- •
- Do not freeze glatiramer acetate syringes. If a syringe freezes, throw it away in a sharps disposal container. See Step 13 in the Instructions for Use, “Dispose of your needles and syringes”.
Keep glatiramer acetate injection and all medicines out of the reach of children.
General information about the safe and effective use of glatiramer acetate injection.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use glatiramer acetate injection for a condition for which it was not prescribed. Do not give glatiramer acetate injection to other people, even if they have the same symptoms as you have. It may harm them. You can ask your pharmacist or healthcare provider for information about glatiramer acetate injection that is written for health professionals.
What are the ingredients in glatiramer acetate injection?
Active ingredient: glatiramer acetate
Inactive ingredients: mannitol
Manufactured for: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 U.S.A.
For more information, go to www.glatirameracetate.com or call 1-844-695-2667.
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: 1/2024
-
Instructions for Use
Glatiramer Acetate Injection
(gla tir′ a mer as′ e tate)
20 mg/mL
for subcutaneous useFor subcutaneous injection only.
Do not inject glatiramer acetate injection in your veins (intravenously).
Do not re-use your glatiramer acetate prefilled syringes.
Do not share your glatiramer acetate prefilled syringes with another person. You may give another person an infection or get an infection from them.
You should receive your first dose of glatiramer acetate injection with a healthcare provider or nurse present. This might be at your healthcare provider’s office or with a visiting home health nurse who will show you how to give your own injections.
Glatiramer acetate injection comes in a 20 mg prefilled syringe with needle attached. How often a dose is given depends on the product strength that is prescribed. Your healthcare provider will prescribe the correct dose for you.
If you plan to use your glatiramer acetate product with an autoinjector, ask your healthcare provider or pharmacist to make sure that your autoinjector is meant to be used with your glatiramer acetate product. If you use an autoinjector that is not meant to be used with your glatiramer acetate product, you might not get the correct dose of your medicine.
Instructions for Using Your Glatiramer Acetate 20 mg Prefilled Syringe:
- •
- Glatiramer acetate injection 20 mg is injected 1 time each day, in the fatty layer under your skin (subcutaneously).
- •
- Each glatiramer acetate 20 mg prefilled syringe is for single use (1 time use) only.
- •
- The glatiramer acetate injection 20 mg dose is packaged in boxes of 30 prefilled syringes with needles attached. Glatiramer acetate 20 mg prefilled syringes have light blue plungers.
How do I inject glatiramer acetate injection?
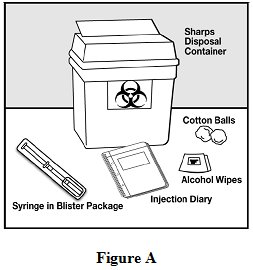
Step 1: Gather the supplies you will need to inject glatiramer acetate injection. See Figure A.
- •
- 1 blister pack with a glatiramer acetate prefilled syringe with needle attached
- •
- Alcohol wipe (not supplied)
- •
- Dry cotton ball (not supplied)
- •
- A place to record your injections, like a notebook (not supplied)
- •
- Sharps disposal container (not supplied). See Step 13 below, “Dispose of your needles and syringes”.
Step 2: Remove only 1 blister pack from the glatiramer acetate prefilled syringe carton. See
Figure B.
- •
- Place the supplies you will need on a clean, flat surface in a well-lit area.
- •
- After you remove 1 blister pack from the carton, keep all unused syringes in the carton and store them in the refrigerator.
- •
- Let the blister pack, with the syringe inside, warm to room temperature for about 20 minutes.
- •
- Wash your hands. Be careful not to touch your face or hair after washing your hands.
Step 3: Look closely at your glatiramer acetate prefilled syringe.
- •
- There may be small air bubbles in the syringe. Do not try to push the air bubble from the syringe before giving your injection so you do not lose any medicine.
- •
- Check the liquid medicine in the syringe before you give your injection. The liquid in the syringe should look clear, and colorless, and may look slightly yellow. If the liquid is cloudy or contains any particles, do not use the syringe and throw it away in a sharps disposal container. See Step 13 below, “Dispose of your needles and syringes.”
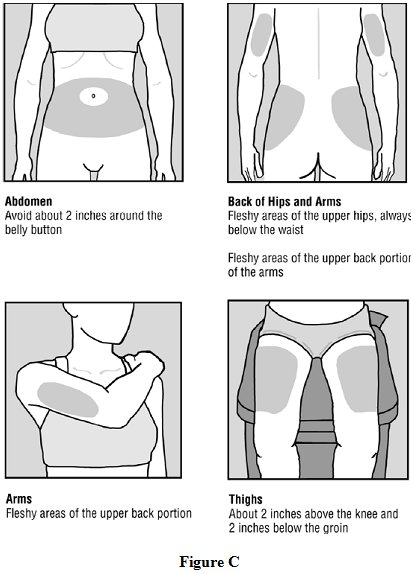
Step 4: Choose your injection area. See Figure C.
See the injection areas you should use on your body. Talk with your healthcare provider about the injection areas that are best for you.
- •
- The possible injection areas on your body include (See Figure C):
- o
- your stomach area (abdomen) around the belly button
- o
- the back of your upper arms
- o
- upper hips (below your waist)
- o
- your thighs (above your knees)
- •
- For each glatiramer acetate injection dose, choose a different injection area from 1 of the areas shown above. See Figure C.
- •
- Do not stick the needle in the same place (site) more than 1 time each week. Each injection area contains multiple injection sites for you to choose from. Avoid injecting in the same site over and over again.
- •
- Keep a record of the sites where you give your injection each day so you will remember where you already injected.
Step 5: Prepare to give your injection.
- •
- There are some injection areas on your body that are hard to reach (like the back of your arm). You may need help from someone who has been instructed on how to give your injection if you cannot reach certain injection areas.
- •
- Do not inject in sites where the skin has scarring or “dents”. Using scarred or dented skin for your injections may make your skin worse.
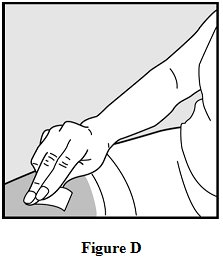
Step 6: Clean your injection site.
- •
- Clean the injection site using the alcohol wipe and allow your skin to air dry. See Figure D.
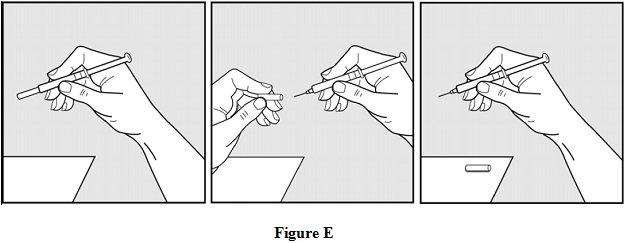
Step 7: Pick up the syringe with 1 hand and hold it like a pencil. Remove the needle cover with your other hand and set it aside. See Figure E.
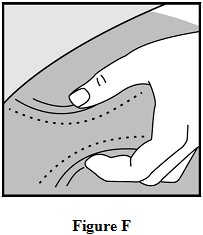
Step 8: Pinch about a 2 inch fold of skin between your thumb and index finger. See Figure F.
Step 9: Giving your injection.
- •
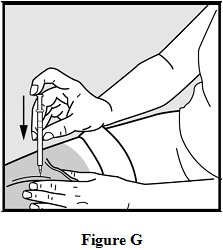
- Rest the heel of your hand holding the syringe against your skin at the injection site. Insert the needle at a 90 degree angle straight into your skin. See Figure G.
- •
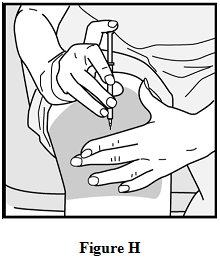
- When the needle is all the way into your skin, release the fold of skin. See Figure H.
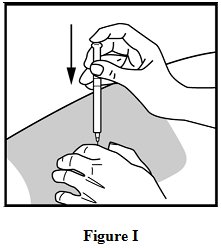
Step 10: Give your glatiramer acetate injection.
To inject the medicine, hold the syringe steady and slowly push down the plunger. See Figure I.
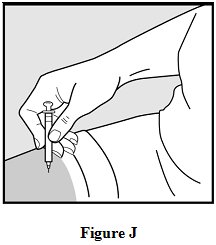
Step 11: Remove the needle.
After you have injected all of the medicine, pull the needle straight out. See Figure J.
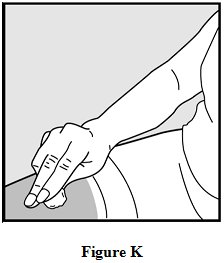
Step 12: Use a clean, dry cotton ball to gently press on the injection site for a few seconds. Do not rub the injection site or re-use the needle or syringe. See Figure K.
Step 13: Dispose of your needles and syringes.
- •
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- upright and stable during use,
- o
- leak-resistant, and
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Manufactured by:
Gland Pharma Ltd
Hyderabad 500 043
IndiaCode No.: AP/DRUGS/10/2012
Revised: 1/2024
GL:GLATIJ:R12
40500425
PSLEA-019456-10 -
PRINCIPAL DISPLAY PANEL – 20 mg/mL
NDC 0378-6960-93
Glatiramer Acetate
Injection
20 mg/mL ONCE DAILYFor Subcutaneous Injection Only
Rx only
Contains 30 Single-Dose PRE-FILLED Syringes
with fixed ½ inch 29 gauge needlesTo be dispensed as a unit package in carton.
Usual Dosage: Inject 20 mg once daily. See accompanying prescribing
|information.Each 1 mL unit-of-use pre-filled syringe
contains:
Active Ingredient: glatiramer acetate 20 mg
Inactive Ingredients: mannitol 40 mg and sterile water for injection.Storage Conditions: KEEP REFRIGERATED (2° to 8°C/36° to 46°F)
AND PROTECT FROM LIGHT.Discard unused portion.
Keep this and all medication out of the reach of children.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Made in India
Code No.: AP/DRUGS/10/2012GL6960:93:30C:R6
Mylan.com
51390001
-
INGREDIENTS AND APPEARANCE
GLATIRAMER ACETATE
glatiramer acetate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0378-6960 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLATIRAMER ACETATE (UNII: 5M691HL4BO) (GLATIRAMER - UNII:U782C039QP) GLATIRAMER ACETATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 40 mg in 1 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (clear, colorless to slightly yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0378-6960-93 30 in 1 CARTON 10/04/2017 1 NDC:0378-6960-32 1 in 1 BLISTER PACK 1 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091646 10/04/2017 Labeler - Mylan Pharmaceuticals Inc. (059295980)